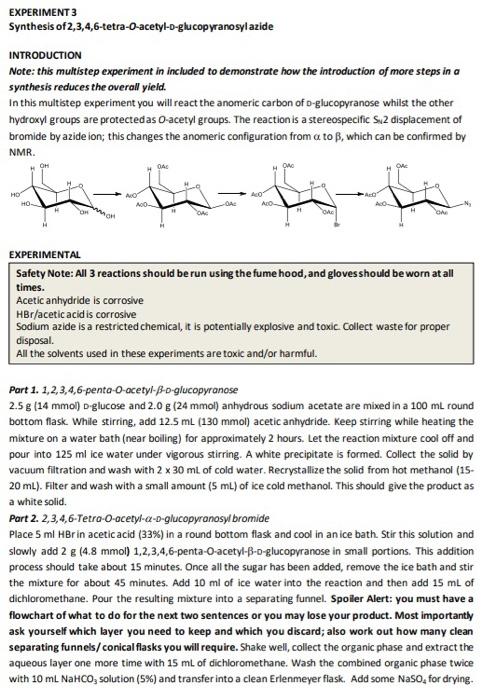
EXPERIMENT 3 Synthesis of 2,3,4,6-tetra-o-acetyl-o-glucopyranosyl azide INTRODUCTION Note: this multistep experiment in included to demonstrate how the introduction of more steps in a synthesis reduces the overall yield. In this multistep experiment you will react the anomeric carbon of D-glucopyranose whilst the other hydroxyl groups are protected as O-acetyl groups. The reaction is a stereospecific S2 displacement of bromide by azide ion; this changes the anomeric configuration from a to B, which can be confirmed by NMR. OM DA ON OA HD HO bi be AO ADO AD AO DH H O EXPERIMENTAL Safety Note: All 3 reactions should be run using the fume hood, and gloves should be worn at all times. Acetic anhydride is corrosive HBr/acetic acid is corrosive Sodium azide is a restricted chemical, it is potentially explosive and toxic Collect waste for proper disposal. All the solvents used in these experiments are toxic and/or harmful. Part 1. 1,2,3,4,6-penta-O-acetyl-Bo-glucopyranose 2.5 g (14 mmol) D-glucose and 2.0 g (24 mmol) anhydrous sodium acetate are mixed in a 100 mL round bottom flask. While stirring, add 12.5 ml (130 mmol) acetic anhydride. Keep stirring while heating the mixture on a water bath (near boiling) for approximately 2 hours. Let the reaction mixture cool off and pour into 125 ml ice water under vigorous stirring. A white precipitate is formed. Collect the solid by vacuum filtration and wash with 2 x 30 ml of cold water. Recrystallize the solid from hot methanol (15- 20 mL). Filter and wash with a small amount (5 mL) of ice cold methanol. This should give the product as a white solid. Part 2. 2,3,4,6-Tetro-O-acetyl-a-D-glucopyranosyl bromide Place 5 ml Brin acetic acid (33%) in a round bottom flask and cool in an ice bath. Stir this solution and slowly add 2 g (4.8 mmol) 1,2,3,4,6-penta-O-acetyl-B-o-glucopyranose in small portions. This addition process should take about 15 minutes. Once all the sugar has been added, remove the ice bath and stir the mixture for about 45 minutes. Add 10 ml of ice water into the reaction and then add 15 mL of dichloromethane. Pour the resulting mixture into a separating funnel. Spoiler Alert: you must have a flowchart of what to do for the next two sentences or you may lose your product. Most importantly ask yourself which layer you need to keep and which you discard; also work out how many clean separating funnels/ conical flasks you will require. Shake well, collect the organic phase and extract the aqueous layer one more time with 15 mL of dichloromethane. Wash the combined organic phase twice with 10 ml NaHCO, solution (5%) and transfer into a clean Erlenmeyer flask. Add some Naso, for drying. Filter or decant the solution into a round bottom flask and remove the dichloromethane with a rotovap; remove a tiny portion and dissolve in chloroform for GC-MS analysis. The product is not stable and will degrade if left exposed to air. It is OK to use for further synthesis within 30 minutes. If it needs to be stored for a longer period, it will have to be stored under an inert atmosphere Flood the flask with a gentle stream of nitrogen for about 2 minutes. Seal it with parafilm and store in the refrigerator/freezer. Start Part 3 of the experiment in this flask but ensure that the flask is warmed to room temperature before opening it. Part 3. 2,3,4,6-Tetra-o-acetyl-B-D-glucopyranosyl azide Dissolve the entire residue from Part 2 or 1 g (2.5 mmol) of 2,3,4,6-tetra-O-acetyl-a -o-glucopyranosyl bromide in 12 mL dimethyl formamide. Add 0.5g(7.5 mmol) sodium azide, fit the flask with a condenser and stir the mixture in a water bath at boiling point for about 2-2.5 hours. Add 12 mL of cold water and transfer into a separating funnel. Spoiler Alert: you must have a flowchart of what to do for the next three sentences or you may lose your product. Most importantly ask yourself which layer you need to keep and which you discard; also work out how many clean separating funnels/conical flasks you will require. Extract this mixture 3 times with 12 mL diethyl ether. Wash the combined organic layers with 12 mL saturated NaCl solution and then with 12 ml water. Pour the organic phase into a clean Erlenmeyer flask and add Na2S0, for drying. Filter or decant into a round bottom flask, and evaporate the solvent under reduced pressure (at the rotovap). This will yield the product as white solid. Record the yield. Similarly to the previous product, the azide is best kept under inert atmosphere and stored in the fridge/freezer. Note: All the reactions can be monitored by TLC with a solvent mixture of hexane: ethyl acetate 1:1. The spots can be visualized by dipping the plate into 5% H2SO, in ethanol and drying it on a hot plate. 4. Characterization Characterize the two intermediates and the final product by GC-MS in chloroform but not the starting material D-glucose (as this is not volatile). Where possible use 'H NMR to identify the signal for the anomeric proton and record its shift and coupling constant. The azide and the bromide are both too unstable to leave for long periods in an NMR tube so you may need to use the spectra given on Moodle for these unless you are able to make up the samples just before acquiring the spectra. Question 1 a) Write a scheme of the preparation indicating the different intermediates and introducing your numbering system? b) Explain why the glucose is fully acetylated in the first step and not just acetylated at C-1? c) Explain why the C-1 acetate is predominantly equatorially configured, why there is a driving force for formation of the axially configured bromide substituent. d) Explain the origin of the stereospecificity of the substitution of bromide by azide? e) Explain using appropriate diagrams how 1H NMR can be used to distinguish the anomeric configuration of D-glucopyranosyl derivatives? Question 1 a) Write a scheme of the preparation indicating the different intermediates and introducing your numbering system? b) Explain why the glucose is fully acetylated in the first step and not just acetylated at C-1? c) Explain why the C-1 acetate is predominantly equatorially configured, why there is a driving force for formation of the axially configured bromide substituent. d) Explain the origin of the stereospecificity of the substitution of bromide by azide? e) Explain using appropriate diagrams how 1H NMR can be used to distinguish the anomeric configuration of D-glucopyranosyl derivatives