Experiment 5: Determination of an Equilibrium Constant
Lab Report Sheet
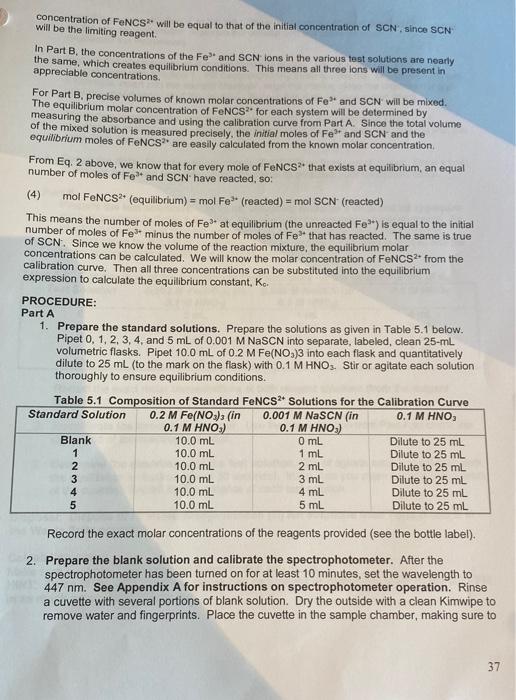
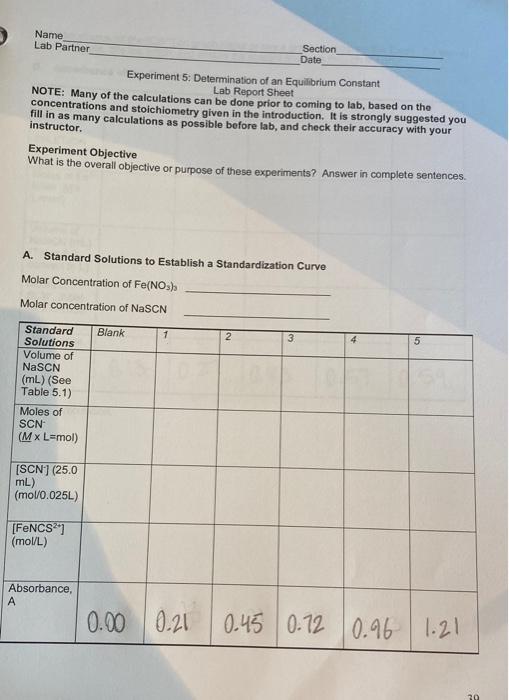
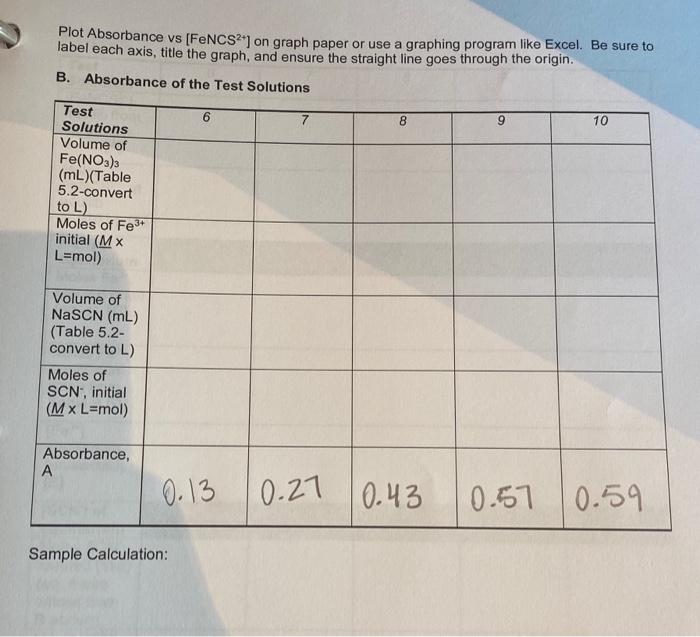
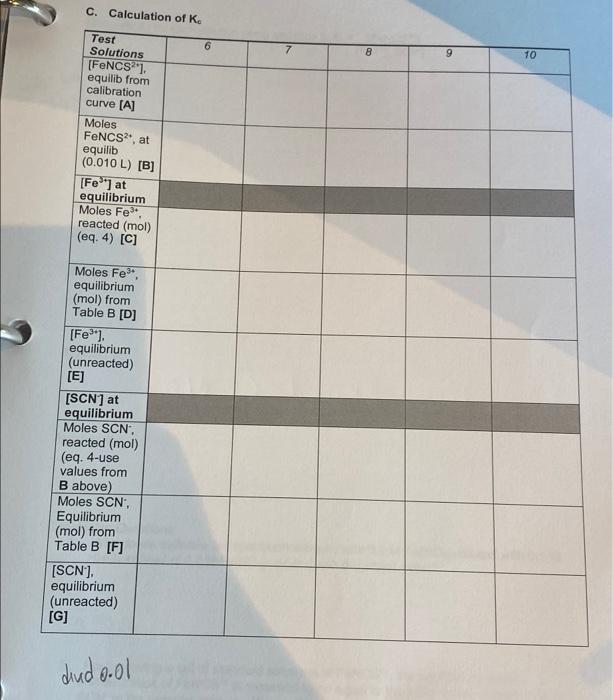
concentration of FeNCS will be equal to that of the initial concentration of SCN, since SCN will be the limiting reagent. In Part B. the concentrations of the Fe and SCN ions in the various test solutions are nearly the same, which creates equilibrium conditions. This means all three lons will be present in appreciable concentrations. For Part B. precise volumes of known molar concentrations of Fe and SCN will be mixed. The equilibrium molar concentration of FeNCS for each system will be determined by measuring the absorbance and using the calibration curve from Part A. Since the total volume of the mixed solution is measured precisely, the initial moles of Fe and SCN and the equilibrium moles of FeNCS2 are easily calculated from the known molar concentration From Eq. 2 above, we know that for every mole of FeNCS that exists at equilibrium, an equal number of moles of Fe and SCN have reacted, so: mol FeNCS? (equilibrium) = mol Fe (reacted) = mol SCN (reacted) This means the number of moles of Feat equilibrium (the unreacted Fe) is equal to the initial number of moles of Fe- minus the number of moles of Fe that has reacted. The same is true of SCN. Since we know the volume of the reaction mixture, the equilibrium molar concentrations can be calculated. We will know the molar concentration of FeNCS from the calibration curve. Then all three concentrations can be substituted into the equilibrium expression to calculate the equilibrium constant, K.. PROCEDURE: Part A 1. Prepare the standard solutions. Prepare the solutions as given in Table 5.1 below. Pipet 0, 1, 2, 3, 4, and 5 mL of 0.001 M NASCN into separate, labeled, clean 25-ml volumetric flasks. Pipet 10.0 mL of 0.2 M Fe(NO3)3 into each flask and quantitatively dilute to 25 ml (to the mark on the flask) with 0.1 M HNO3. Stir or agitate each solution thoroughly to ensure equilibrium conditions. Table 5.1 Composition of Standard FeNCS2Solutions for the Calibration Curve Standard Solution 0.2 M Fe(NO3), (in 0.001 M NaSCN (in 0.1 M HNO, 0.1 M HNO.) 0.1 M HNO:) Blank 10.0 mL O mL Dilute to 25 mL 1 10.0 mL 1 ml Dilute to 25 mL 2 10.0 mL 2 mL Dilute to 25 mL 3 10.0 mL 3 mL Dilute to 25 ml 4 10.0 mL 4 mL Dilute to 25 mL 5 10.0 ml 5 mL Dilute to 25 mL Record the exact molar concentrations of the reagents provided (see the bottle label). 2. Prepare the blank solution and calibrate the spectrophotometer. After the spectrophotometer has been turned on for at least 10 minutes, set the wavelength to 447 nm. See Appendix A for instructions on spectrophotometer operation. Rinse a cuvette with several portions of blank solution. Dry the outside with a clean Kimwipe to remove water and fingerprints. Place the cuvette in the sample chamber, making sure to 37 align the cuvette with the V to the left. Close the chamber lid, and zero the absorbance on the spectrophotometer. Remove the cuvette and put aside (in case you need to recalibrate the instrument) 3. Record the absorbance of the standard solutions. Using a clean cuvette, rinse several times with Solution 1. Fill it approximately three-fourths full. Dry the outside with a kimwipe, insert into the sample chamber, align the marks, close the chamber lid and read the absorbance off of the readout. Record this data on your report sheet. Depending on availability, you may use one cuvette for each standard solution. If cuvettes are in short supply, you can use one cuvette for each standard, starting with the most dilute concentration 4. Graph the data. Plot absorbance versus (FeNCS2"] on linear graph paper. Draw a best fit straight line (trend line) through the six points to establish your calibration curve. Part B 1. Prepare the test solutions. In 10 mL volumetric flasks (or a large test tube) prepare the test solutions in Table 5.2 below. Use pipets for the volumetric measurements. Be careful not to mix pipet to avoid contamination of the reagents. Also note that the concentrations for this set of solutions is 0.002 M, not the 0.2 M used in Part A, and the molar concentration of NaSCN is 0.002 M, not 0.001 M Table 5.2. Composition of the Equilibrium Test Solutions for the Determination of Ke Test Solution 0.002 M Fe(NO), (in 0.002 M NaSCN (in 0.1 M HNO 0.1 M HNO) 0.1 M HNO) 6 5 ml 1 ml 4 mL 7 5 mL 2 mL 3 mL 8 5 ml 3 mL 2 mL 9 5 mL 4 mL 1 mL 10 5 ml 5 ml Record the exact molar concentrations of both reagent solutions (from the reagent bottle). 2. Recalibrate the spectrophotometer. Use the blank solution from Part A to check the calibration of the spectrophotometer. 3. Determine the absorbance of the test solution. Stir or agitate each test solution until the equilibrium is reached (about 30 seconds). Make sure you clean and dry the outside of the cuvette. Record the absorbance of each test solution Disposal: Dispose of all waste thiocyanatoiron (II) ion solutions from Parts A and B in the Waste Salts container. Calculation notes: Make sure you know how to convert concentration into moles. HINT: Some of the data for these tables can be entered or calculated prior to the lab. Fill in as much as possible before lab. 29 Name Lab Partner Section Date Experiment 5: Determination of an Equilibrium Constant Lab Report Sheet NOTE: Many of the calculations can be done prior to coming to lab, based on the concentrations and stoichiometry given in the introduction. It is strongly suggested you fill in as many calculations as possible before lab, and check their accuracy with your instructor. Experiment Objective What is the overall objective or purpose of these experiments? Answer in complete sentences. A. Standard Solutions to Establish a Standardization Curve Molar Concentration of Fe(NO) Molar concentration of NSCN Blank 1 2 3 5 Standard Solutions Volume of NaSCN (mL) (See Table 5.1) Moles of SCN (Mx L=mol) (SCN) (25.0 mL) (mol0.025L) [FeNCS) (mol/L) Absorbance, A 0.00 0.21 0.21 0.45 0.72 0.96 1.21 Plot Absorbance vs (FeNCS2+) on graph paper or use a graphing program like Excel. Be sure to label each axis, title the graph, and ensure the straight line goes through the origin. B. Absorbance of the Test Solutions 6 7 8 9 10 Test Solutions Volume of Fe(NO3)3 (ML)(Table 5.2-convert to L) Moles of Fe3+ initial (MX L=mol) Volume of NaSCN (mL) (Table 5.2- convert to L) Moles of SCN: initial (M x L=mol) Absorbance, A 0.13 0.27 0.43 0.57 0.59 Sample Calculation: C. Calculation of K. 6 8 9 10 Test Solutions [FeNCS"), equilib from calibration curve [A] Moles FeNCS2, at equilib (0.010 L) [B] [Fe] at equilibrium Moles Fes- reacted (mol) (eq. 4) [C] Moles Fe. equilibrium (mol) from Table B [D] [Fe]. equilibrium (unreacted) [E] [SCN) at equilibrium Moles SCN. reacted (mol) (eq. 4-use values from B above) Moles SCN, Equilibrium (mol) from Table B [F] [SCN). equilibrium (unreacted) [G] dindoool Calculate Ke for each solution using equation 3.K - IFENCS [Fe 'SCN) Solution 6: value bb E6XG6 Solution 7: Solution 8: Solution 9 Solution 10: Average Kc: Concluding Questions: 1. Why do we calibrate the spectrophotometer with 0.2 M Fe(NO3) diluted with 0.1 M HNO, instead of 0.2 M Fe(NO3), diluted with deionized water? 2. For preparing a set of standard solutions of FeNCS, the equilibrium molar concentration of FeNCS2 is assumed to be equal to the initial molar concentration of the SCN in the reaction mixture. Why is this assumption valid? 42