Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Experiment #6. Iodine Clock Reaction Part 2 In This Experiment You Will Determine The Rate Law For The Following Oxidation-Reduction Reaction: 2H+ (Aq) 2?(Aq) H202
Experiment #6. Iodine Clock Reaction Part 2 In This Experiment You Will Determine The Rate Law For The Following Oxidation-Reduction Reaction: 2H+ (Aq) 2?(Aq) H202 (Aq) 12(Aq) + 2H20 (I) + + ? The Rate Or Speed Of The Reaction Is Dependent On The Concentrations Of Iodide Ion (I) And Hydrogen Peroxide H02. (The Spectator Ions Are Left Off The Reaction.)

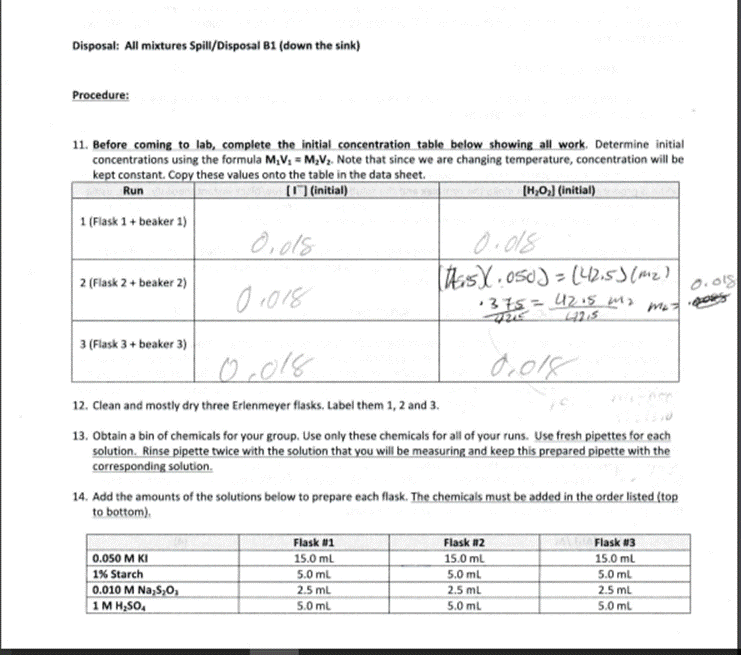
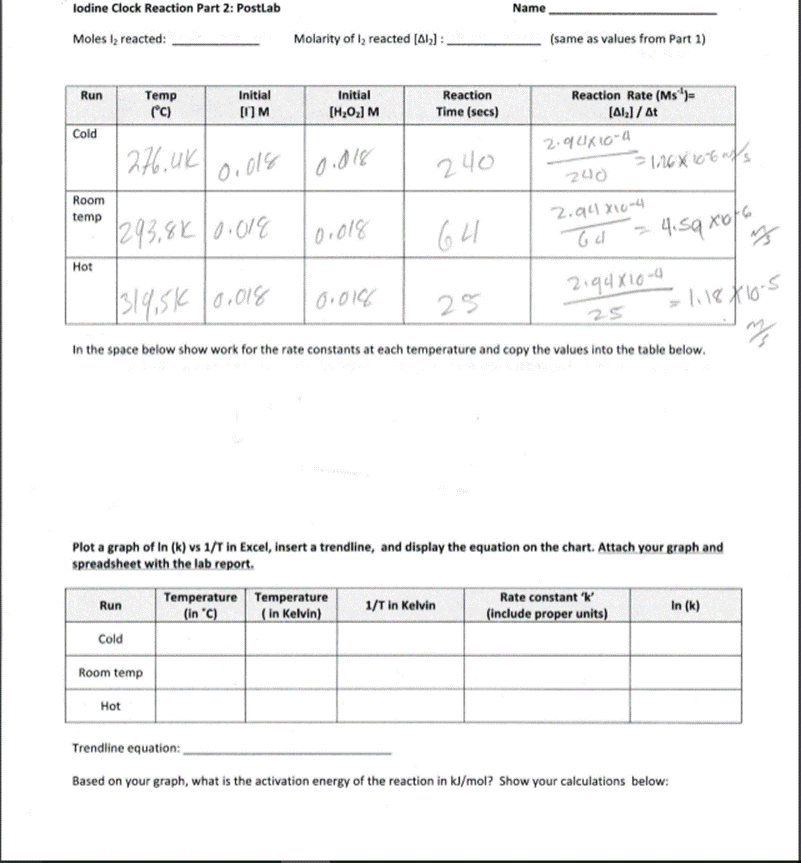
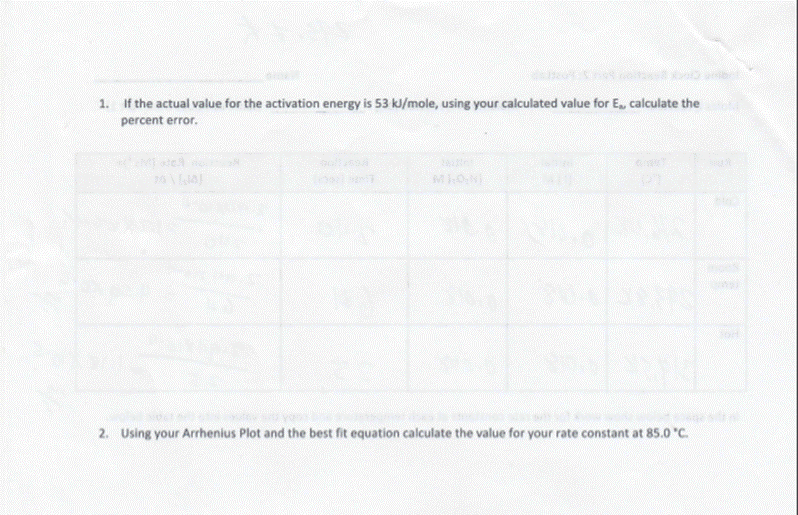
Introduction Experiment #6. Iodine Clock Reaction Part 2 In this experiment you will determine the Rate Law for the following oxidation-reduction reaction: 2 H(aq) + 2(aq) + H2O2(aq) 12 (aq) + 2 HO (1) (1) The rate or speed of the reaction is dependent on the concentrations of iodide ion (I) and hydrogen peroxide, HO2 (The spectator ions are left off the reaction.) Therefore, we can write the Rate Law (concentration dependence) for the reaction as: Rate = k [1] [HO] (2) The temperature dependence of the rate is seen in k - that is, there is a separate value of k for each temperature at which the reaction takes place. As with a lot of kinetics, the concentration of reactants or products at any instant is difficult to measure directly, so in this lab the rate will be determined indirectly. We have a very handy test for the presence of one of the products, lodine (2), namely starch. Starch reacts with iodine to form a blue/black colored complex. Unfortunately as soon as any iodine is produced it will react to make the complex and the solution will turn blue/black instantaneously. Thus, using starch as an indicator by itself would not be of much help. It confirms that some amount of 12 is being formed, but it tells us nothing about what we are trying to measure - the rate (how long it takes to produce a given quantity of l.) To get around this problem we will introduce a side reaction that will remove the initial l that is produced by our main reaction. This will prevent the solution from turning black long enough so that we can make some time measurements. We will use the following side reaction: Iz (aq) + 2 SO, (aq) 21 (aq) + SO2 (aq) (3) SO,,thiosulfate ion, reacts with l which prevents the solution from turning blue/black. How will this help? Since we have carefully measured the amount of thiosulfate (a small amount that will run out fairly quickly), we know exactly how much iodine it will take to react with this thiosulfate. Once the small amount thiosulfate has completely reacted, I will start to build up in the solution. As soon as the thiosulfate runs out, I will react with the starch and the solution will turn blue/black. By putting in this "time delay", we can now calculate the rate at which I is being formed. The rate of reaction is equal to the change in concentration divided by the change in time. The change in time will be the time it takes for the solution to turn dark. We will calculate the change in concentration in l based on the amount of thiosulfate added. Using the known volume and molarity, we can calcuate moles of thiosulfate (S203). Based on stoichiometry, we can calculate the moles of 12: according to equation (3), 2 moles of thiosulfate react with every 1 mole of l, This gives us the change in moles, however for the rate formula we need change in concentration. Divide the moles of 12 reacted by the total volume to find the change in molarity. Rate=[Al]/At (4)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


