Question
Experimental Determination of AG for ATP Hydrolysis A direct measurement of the standard free-energy change associated with the hydrolysis of ATP is technically demanding
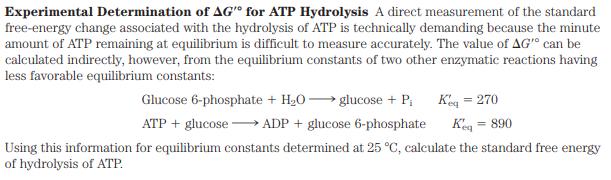
Experimental Determination of AG" for ATP Hydrolysis A direct measurement of the standard free-energy change associated with the hydrolysis of ATP is technically demanding because the minute amount of ATP remaining at equilibrium is difficult to measure accurately. The value of AG" can be calculated indirectly, however, from the equilibrium constants of two other enzymatic reactions having less favorable equilibrium constants: Glucose 6-phosphate + HO glucose + P Keq = 270 ATP + glucoseADP + glucose 6-phosphate Keq = 890 Using this information for equilibrium constants determined at 25 C, calculate the standard free energy of hydrolysis of ATP.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry
Authors: Darrell Ebbing, Steven D. Gammon
9th edition
978-0618857487, 618857486, 143904399X , 978-1439043998
Students also viewed these Biology questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



