Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Use the rate of chemical reaction data provided in the experiment and tables below to complete the tables in problem 3: ? Experimental Procedure 50.0
Use the rate of chemical reaction data provided in the experiment and tables below to complete the tables in problem 3:
![]()
 ?
?
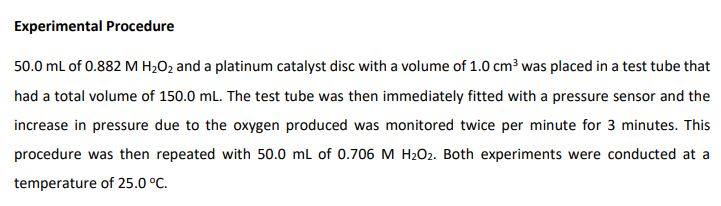
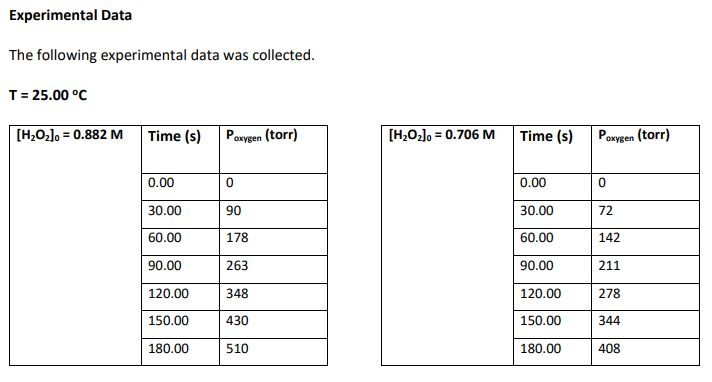
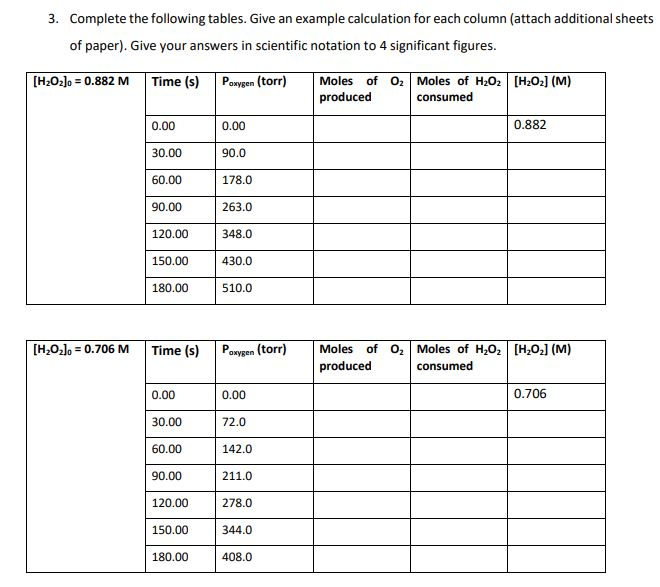
Experimental Procedure 50.0 mL of 0.882 M HO and a platinum catalyst disc with a volume of 1.0 cm was placed in a test tube that had a total volume of 150.0 mL. The test tube was then immediately fitted with a pressure sensor and the increase in pressure due to the oxygen produced was monitored twice per minute for 3 minutes. This procedure was then repeated with 50.0 mL of 0.706 M HO2. Both experiments were conducted at a temperature of 25.0 C. 2H2O2(aq) O2(g) + 2HO (1) Experimental Data The following experimental data was collected. T = 25.00 C [HO] = 0.882 M Time (s) P 0.00 30.00 60.00 90.00 120.00 150.00 180.00 oxygen (torr) 0 90 178 263 348 430 510 [HO] = 0.706 M Time (s) 0.00 30.00 60.00 90.00 120.00 150.00 180.00 P oxygen (torr) 0 72 142 211 278 344 408 3. Complete the following tables. Give an example calculation for each column (attach additional sheets of paper). Give your answers in scientific notation to 4 significant figures. [HzOz]o = 0.882 M Time (s) Poxygen (torr) [HO]0= 0.706 M 0.00 30.00 60.00 90.00 120.00 150.00 180.00 Time (s) 0.00 30.00 60.00 90.00 120.00 150.00 180.00 0.00 90.0 178.0 263.0 348.0 430.0 510.0 Paxygen (torr) 0.00 72.0 142.0 211.0 278.0 344.0 408.0 Moles of O Moles of HO [HO] (M) produced consumed 0.882 Moles of O Moles of HO [H0] (M) produced consumed 0.706
Step by Step Solution
★★★★★
3.40 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Given that the volume of the test tube 150 mL The volume of H2O2 volume of platinum disc 50 1 51 mL ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


