Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Experiments were made on a bottom-hole sample of the reservoir liquid taken from the lasaale oil field to determine the solution gas and formation
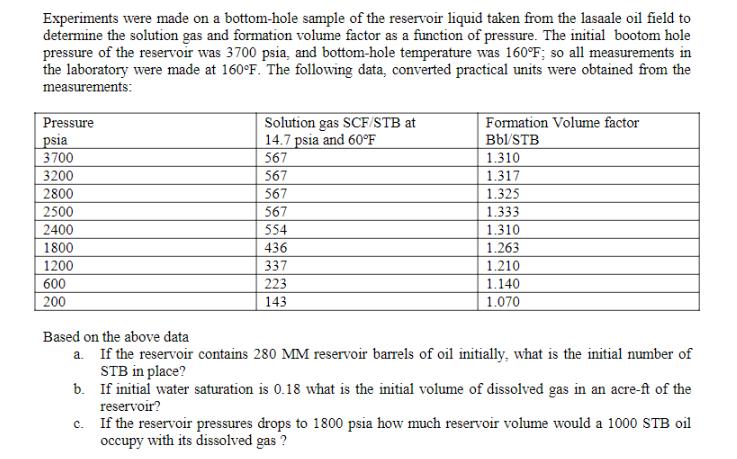
Experiments were made on a bottom-hole sample of the reservoir liquid taken from the lasaale oil field to determine the solution gas and formation volume factor as a function of pressure. The initial bootom hole pressure of the reservoir was 3700 psia, and bottom-hole temperature was 160F; so all measurements in the laboratory were made at 160F. The following data, converted practical units were obtained from the measurements: Solution gas SCF/STB at 14.7 psia and 60F 567 Pressure Formation Volume factor _psia 3700 Bbl/STB 1.310 3200 2800 567 1.317 567 1.325 2500 2400 567 1.333 554 1.310 1800 436 1.263 1200 337 1.210 600 223 1.140 200 143 1.070 Based on the above data a. If the reservoir contains 280 MM reservoir barrels of oil initially, what is the initial number of STB in place? b. If initial water saturation is 0.18 what is the initial volume of dissolved gas in an acre-ft of the reservoir? c. If the reservoir pressures drops to 1800 psia how much reservoir volume would a 1000 STB oil occupy with its dissolved gas ?
Step by Step Solution
★★★★★
3.30 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1
first lets observe the bubble point from the given data in order to make further calculations we can ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


