Answered step by step
Verified Expert Solution
Question
1 Approved Answer
explain and show all work please answer all parts A to D or I will give you a thumbs down and report Matlab part D
explain and show all work please answer all parts A to D or I will give you a thumbs down and report Matlab part D
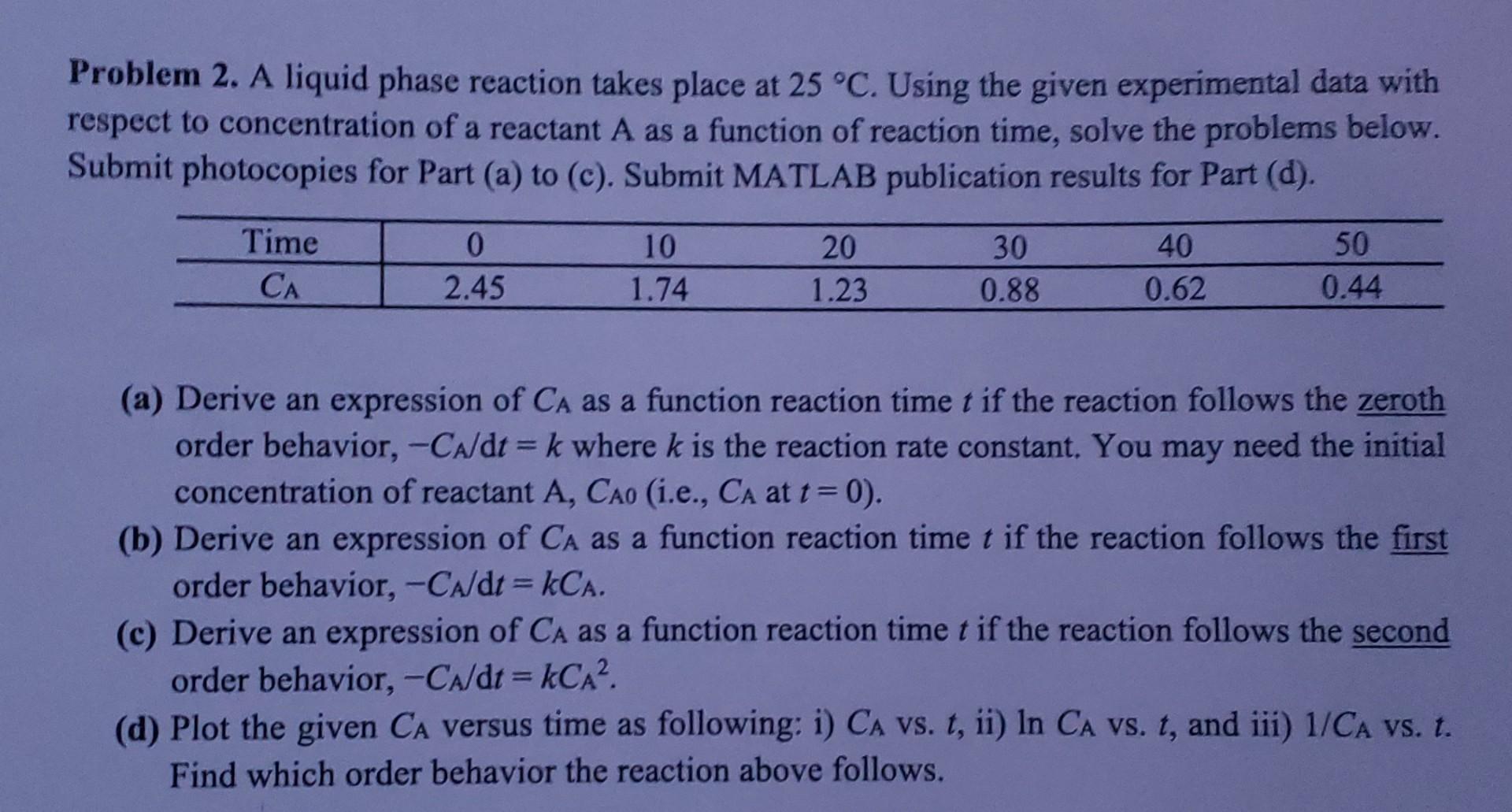
Problem 2. A liquid phase reaction takes place at 25C. Using the given experimental data with respect to concentration of a reactant A as a function of reaction time, solve the problems below. Submit photocopies for Part (a) to (c). Submit MATLAB publication results for Part (d). (a) Derive an expression of CA as a function reaction time t if the reaction follows the zeroth order behavior, CA/dt=k where k is the reaction rate constant. You may need the initial concentration of reactant A,CA0 (i.e., CA at t=0 ). (b) Derive an expression of CA as a function reaction time t if the reaction follows the first order behavior, CA/dt=kCA. (c) Derive an expression of CA as a function reaction time t if the reaction follows the second order behavior, CA/dt=kCA2. (d) Plot the given CA versus time as following: i) CA vs. t, ii) lnCA vs. t, and iii) 1/CA vs. t. Find which order behavior the reaction above followsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


