Answered step by step
Verified Expert Solution
Question
1 Approved Answer
explain how to do formal charge on a atom. empahsis on letters L, M, N A. What is the formal charge on the oxygen in
explain how to do formal charge on a atom. empahsis on letters L, M, N 
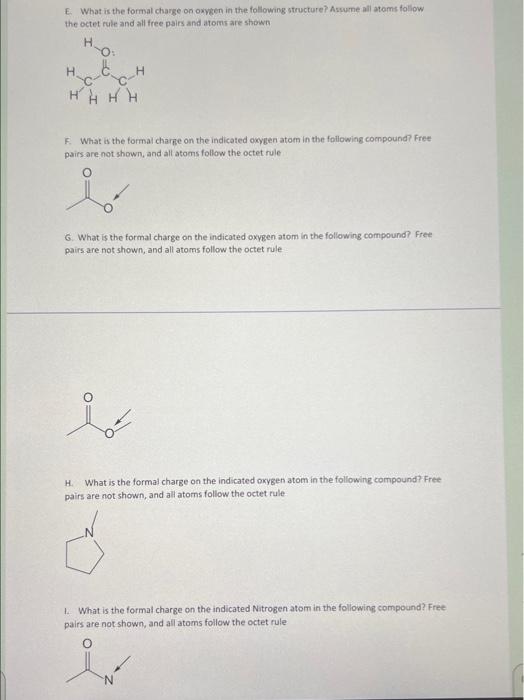
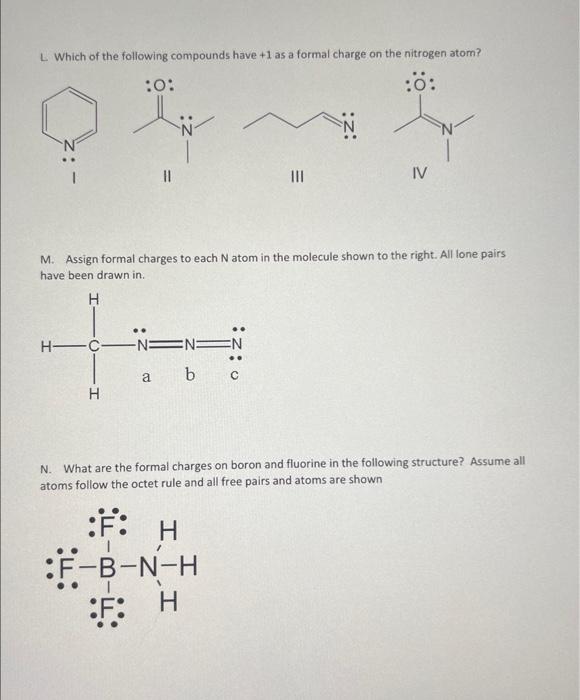
A. What is the formal charge on the oxygen in the following compound? B. What is the formal charge on oxygen in the following structure? Assume all atoms foliow the octet rule and all free pairs and atoms are shown C. What is the formal charge on the nitrogen indicated in the following compound?: D. What is the formal charge on nitrogen in the following structure? Assume all atoms follow the octet rule and all free pairs and atoms are shown E. What is the formal charge on oxyen in the following structure? Assume all atoms follow the octet rule and all free pairs and atoms are shown F. What is the formal charge on the indicated oxygen atom in the following compound? Free pairs are not shown, and all atoms follow the octet rule 6. What is the formal charge on the indieated oxygen atom in the following compound? Free pairs are not shown, and all atoms follow the octet rule H. What is the formal charge on the indicated oxygen atom in the following compound? Free pairs are not shown, and all atoms follow the octet rule. 1. What is the formal charge on the indicated Nitrogen atom in the following compound? Free pairs are not shown, and all atoms follow the octet rule L. Which of the following compounds have +1 as a formal charge on the nitrogen atom? II IV M. Assign formal charges to each N atom in the molecule shown to the right. All lone pairs have been drawn in. N. What are the formal charges on boron and fluorine in the following structure? Assume all atoms follow the octet rule and all free pairs and atoms are shown 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


