Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Explain how to find the fractional abundance/atomic mass hg? Results 1. Mercury peaks Mass Number 198 Measurement (cm) 1. Calculate the fractional abundances of the
Explain how to find the fractional abundance/atomic mass hg?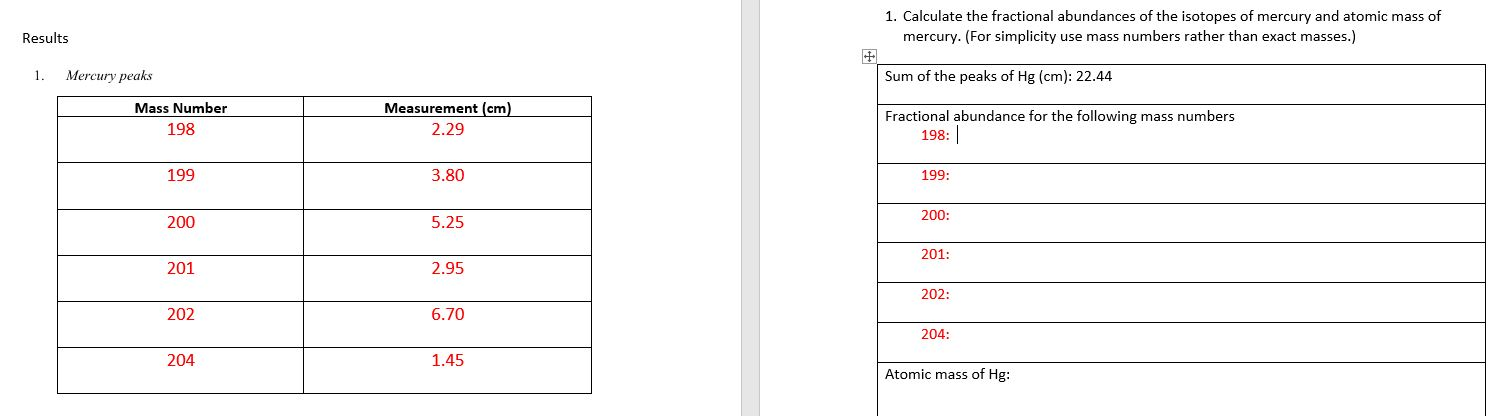
Results 1. Mercury peaks Mass Number 198 Measurement (cm) 1. Calculate the fractional abundances of the isotopes of mercury and atomic mass of mercury. (For simplicity use mass numbers rather than exact masses.) Sum of the peaks of Hg (cm): 22.44 Fractional abundance for the following mass numbers 2.29 198: | 199 3.80 199: 200 5.25 200: 201: 201 2.95 202: 202 6.70 204: 204 1.45 Atomic mass of Hg:
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The fractional abundance of an isotope refers to the proportion of that isotope within a sample of a...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
6642fc0d35ad1_960469.pdf
180 KBs PDF File
6642fc0d35ad1_960469.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


