Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Explain why the first ionization energy of Ca is greater than that of K, whereas the second ionization energy of Ca is lower than
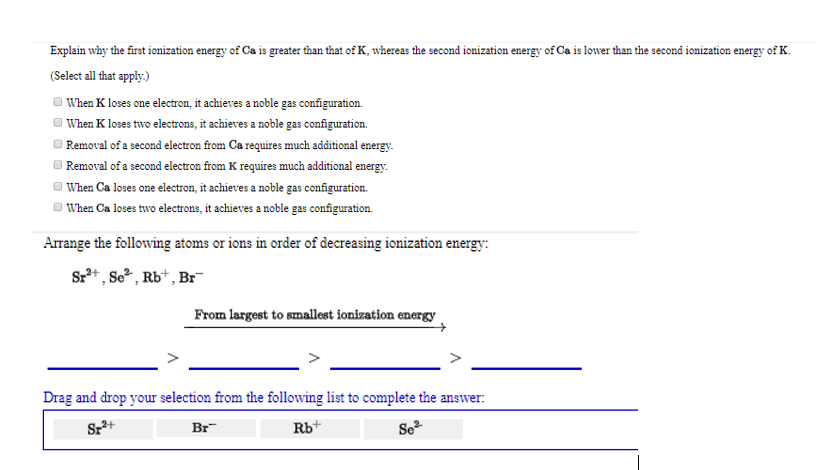
Explain why the first ionization energy of Ca is greater than that of K, whereas the second ionization energy of Ca is lower than the second ionization energy of K. (Select all that apply.) When K loses one electron, it achieves a noble gas configuration. When K loses two electrons, it achieves a noble gas configuration. Removal of a second electron from Ca requires much additional energy. Removal of a second electron from K requires much additional energy. When Ca loses one electron, it achieves a noble gas configuration. When Ca loses two electrons, it achieves a noble gas configuration. Arrange the following atoms or ions in order of decreasing ionization energy: Sr+, So, Rb+, Br From largest to smallest ionization energy Drag and drop your selection from the following list to complete the answer: Sz+ Br Rb+ Se
Step by Step Solution
★★★★★
3.57 Rating (175 Votes )
There are 3 Steps involved in it
Step: 1
Answer 1 The first ionization energy of an element is the energy required to remove one electron from a neutral atom of that element Calcium has an at...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


