Question
Explain why water is polar. Check all that apply. View Available Hint(s) Water is known as a polar molecule because the electrons of the
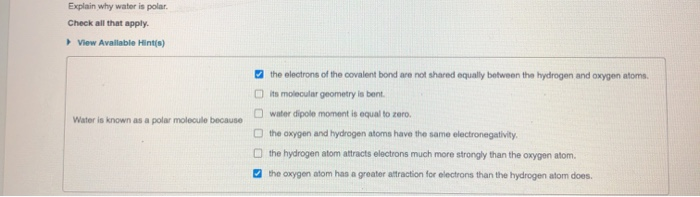
Explain why water is polar. Check all that apply. View Available Hint(s) Water is known as a polar molecule because the electrons of the covalent bond are not shared equally between the hydrogen and oxygen atoms. its molecular geometry is bent. water dipole moment is equal to zero. the oxygen and hydrogen atoms have the same electronegativity. the hydrogen atom attracts electrons much more strongly than the oxygen atom. the oxygen atom has a greater attraction for electrons than the hydrogen atom does.
Step by Step Solution
3.58 Rating (173 Votes )
There are 3 Steps involved in it
Step: 1
Water is a polar molecule because it has a net dipole moment Wate...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Basic Marketing Research
Authors: Tom J. Brown, Tracy A. Suter, Gilbert A. Churchill
8th edition
1133188540, 978-1111525293, 1111525293, 978-1305178571, 1305178572, 978-1133188544
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



