Question
Expt. 2 Preparation and Standardization of Solutions for Acidimetry and Alkalimetry DATA SHEET A. Standardization of prepared NaOH solution Data & Results: Complete the table.
Expt. 2 Preparation and Standardization of Solutions for Acidimetry and Alkalimetry
DATA SHEET
A. Standardization of prepared NaOH solution
Data & Results: Complete the table.
|
| TRIAL 1 | TRIAL 2 | TRIAL 3 |
| Wt. of KHP (MM 204 g/mol) | 0.3580 g | 0.3650 | 0.3600 |
| Titration Data |
|
|
|
| Final Reading | 7.50 mL | 14.70 mL. | 22.00 mL |
| Initial Reading | 0.00 mL | 7.50 mL | 14.70 mL |
| Total vol. of NaOH used | ________ | _________ | _______ |
| Std. Concn . of NaOH, (N) | ________ | _________ | _______ |
| Ave. Std. Concn. of NaOH | ________ | ||
B. Standardization of the prepared HCl solution
Data & Results: Complete the table.
|
| TRIAL 1 | TRIAL 2 | TRIAL 3 |
| Vol. of HCl used | 20.00 mL | 20.00 mL | 20.00 mL |
| Titration Data |
|
|
|
| Final Reading | 19.00 | 18.80 | 19.20 |
| Initial Reading | 0.00 mL | 0.00 mL | 0.00 mL |
| Total vol. of NaOH used | _______ | _______ | _______ |
| Std. Concn . of HCl (N) | _______ | _______ | _______ |
| Ave. Std. Concn. of HCl | ________ | ||
NOTE: All weight readings from analytical balance must be expressed up to 4 decimal places. All volume readings must be expressed up to 2 decimal places.
Guide Questions
- Give the general directions for the use of volumetric flask in the preparation of accurate volume solution.
____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
- Write the equation showing how to determine the calculated concentration of NaOH:
___________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
of HCl: ________________________________________________________________________
_______________________________________________________________________________
- What is the appropriate indicator in the titration of a strong acid with a strong base? ____________________________ of a weak acid with a strong base? ______________________
- How do you differentiate the method of standardizing the NaOH solution from that of HCl solution? _______________________________________________________________________
_______________________________________________________________________________
- What is a primary standard substance? _______________________________________________
_______________________________________________________________________________
(this is only a guideline on how to compute)
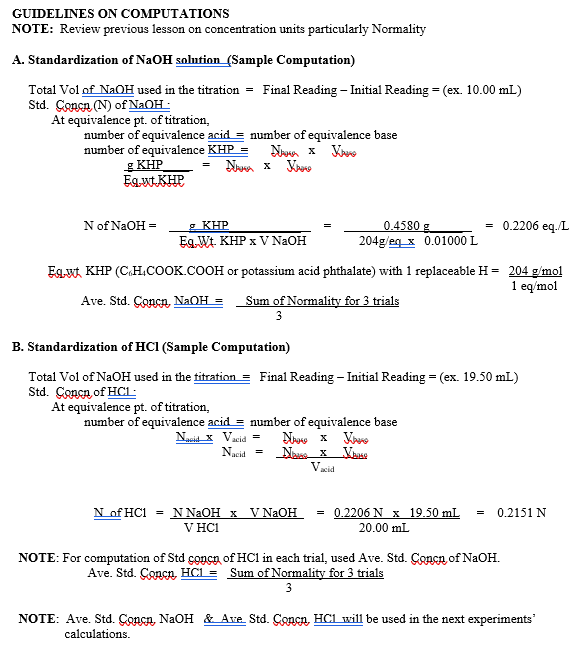
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


