Answered step by step
Verified Expert Solution
Question
1 Approved Answer
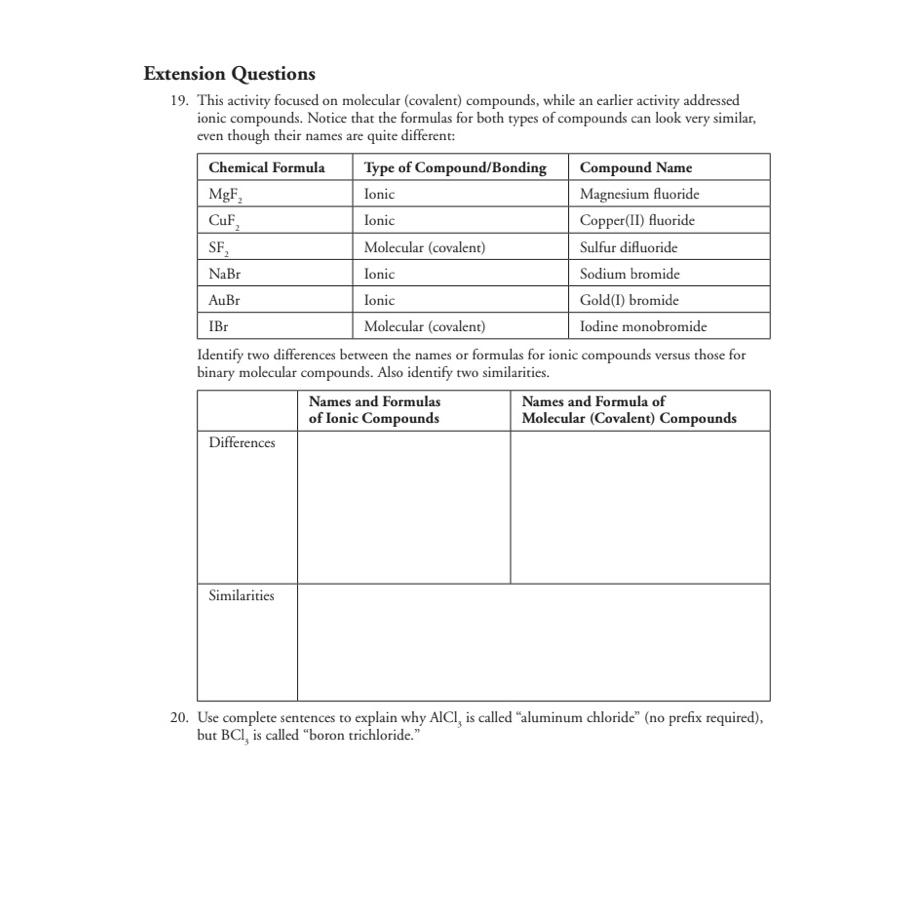
Extension Questions 1 9 . This activity focused on molecular ( covalent ) compounds, while an earlier activity addressed ionic compounds. Notice that the formulas
Extension Questions
This activity focused on molecular covalent compounds, while an earlier activity addressed ionic compounds. Notice that the formulas for both types of compounds can look very similar, even though their names are quite different:
tableChemical Formula,Type of CompoundBondingCompound NameIonic,Magnesium fluorideIonic,CopperII fluorideMolecular covalentSulfur difluorideIonic,Sodium bromideIonic,GoldI bromideMolecular covalentIodine monobromide
Identify two differences between the names or formulas for ionic compounds versus those for binary molecular compounds. Also identify two similarities.
tabletableNames and Formulasof Ionic CompoundstableNames and Formula ofMolecular Covalent CompoundsDifferencesSimilarities
Use complete sentences to explain why is called "aluminum chloride" no prefix required but is called "boron trichloride."
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


