Question: a. Construct a model for CH, (methane) by adding a ball representing hydrogen to each of the four bonds of your carbon model. Draw the
a. Construct a model for CH, (methane) by adding a ball representing hydrogen to each of the four bonds of your carbon model. Draw the full structural formula represented by this model.
b. Remove one of the hydrogen atoms from your methane model and replace it with a chlorine atom. ibis is chloro methane. Draw the full structural formula.
c. Make another model of methane and replace a different hydrogen with chlorine to make a second model of chloro methane. Are the two models the same or different?
d. What can be said about the relationship (same structure or different?) between the molecules represented by the following full structural formulas?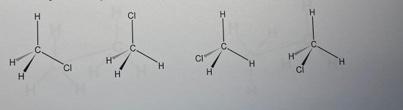
e. Write can be said about relation between the molecule of methodicalness represent below?
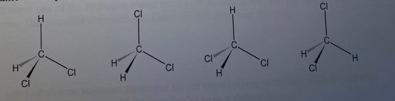
f. Write the condensed formula for dichromethane.
Step by Step Solution
3.36 Rating (152 Votes )
There are 3 Steps involved in it
Lets go through each part of the question a Methane Model and Structural Formula Methane CH consists ... View full answer

Get step-by-step solutions from verified subject matter experts


