Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Using the energy level chart below, if a photon with an energy of 2.856 eV is * 1 point emitted from this atom what
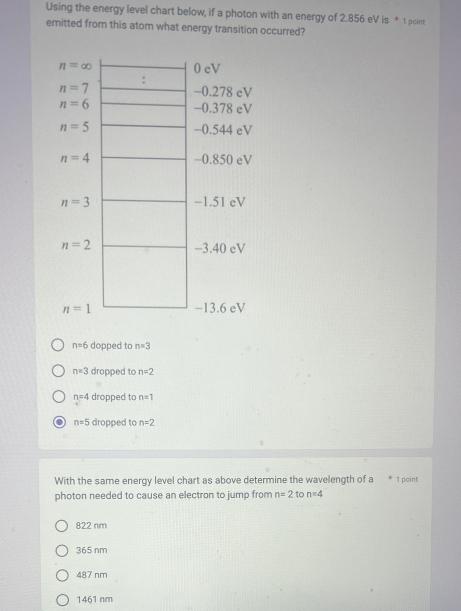
Using the energy level chart below, if a photon with an energy of 2.856 eV is * 1 point emitted from this atom what energy transition occurred? 818 n=7 n=5 n=4 n=3 n=2 n=1 n=6 dopped to n=3 ne3 dropped to n=2 n=4 dropped to n=1 n=5 dropped to n=2 822 nm 365 nm 487 nm 0 cV -0.278 eV -0.378 eV -0.544 eV -0.850 eV With the same energy level chart as above determine the wavelength of a photon needed to cause an electron to jump from n=2 ton=4 1461 nm -1.51 eV -3.40 eV -13.6 eV 1 point
Step by Step Solution
★★★★★
3.46 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
To determine the energy transition that occurred when a photon with an energy of 2856 eV was emitted ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


