Question: The energy-level scheme for the hypothetical one electron element Searsium is shown in Fig. E39.25. The potential energy is taken to be zero for an
The energy-level scheme for the hypothetical one electron element Searsium is shown in Fig. E39.25. The potential energy is taken to be zero for an electron at an infinite distance from the nucleus.
(a) How much energy (in electron volts) does it take to ionize an electron from the ground level?
(b) An 18-eV photon is absorbed by a Searsium atom in its ground level. As the atom returns to its ground level, what possible energies can the emitted photons have? Assume that there can be transitions between all pairs of levels.
(c) What will happen if a photon with an energy of 8 eV strikes a Searsium atom in its ground level? Why?
(d) Photons emitted in the Searsium transitions n = 3 †’ n = 2 and n = 3 †’ n = 1 will eject photoelectrons from an unknown metal, but the photon emitted from the transition n = 4 †’ n = 3 will not. What are the limits (maximum and minimum possible values) of the work function of the metal?
Figure E39.25
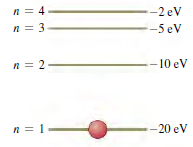
-2 eV -5 eV -10 eV -20 eV
Step by Step Solution
3.48 Rating (165 Votes )
There are 3 Steps involved in it
IDENTIFY and SET UP The ionization threshold is at E 0 The energy of an absorbed photon equals the e... View full answer

Get step-by-step solutions from verified subject matter experts


