Answered step by step
Verified Expert Solution
Question
1 Approved Answer
fast Q1. (10 marks) Consider the cell Zn(s)+Ag2O(s)+H2O(t)Zn2+(aq)+2Ag(s)+2OH(aq)E0=1.098V (a) Write the electrodes half-reactions with the standard potential for both. ( 2 marks) (b) Calculate the
fast 
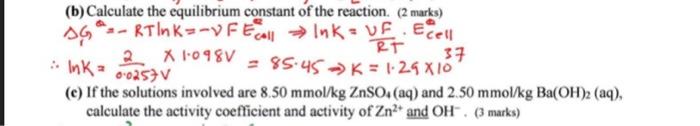
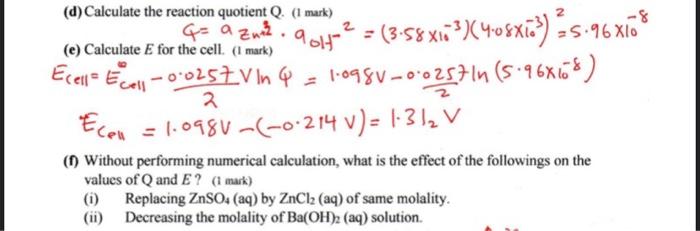
Q1. (10 marks) Consider the cell Zn(s)+Ag2O(s)+H2O(t)Zn2+(aq)+2Ag(s)+2OH(aq)E0=1.098V (a) Write the electrodes half-reactions with the standard potential for both. ( 2 marks) (b) Calculate the equilibrium constant of the reaction. ( 2 marks) Ga=RTlnK=FEcellelnK=RTUF.ECe11AlnK=0.0257V21.098V=85.45K=1.241037 (c) If the solutions involved are 8.50mmol/kgZnSO4 (aq) and 2.50mmol/kgBa(OH)2(aq), calculate the activity coefficient and activity of Zn2+ and OH.(3 marks) (d) Calculate the reaction quotient Q. (1 mark) (e) Calculate E for the cell. (1 mark) a01H=(3.58103)(4.08103)2=5.96 E(e1)=ECe1120.0257Vln=1.098V20.0257ln(5.96108)ECel=1.098V(0.214V)=1.312V (f) Without performing numerical calculation, what is the effect of the followings on the values of Q and E ? (1 mark) (i) Replacing ZnSO4 (aq) by ZnCl2 (aq) of same molality. (ii) Decreasing the molality of Ba(OH)2 (aq) solution 
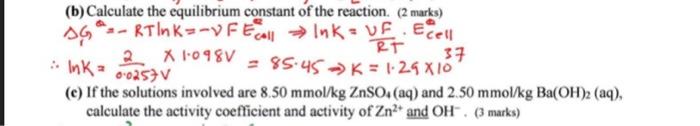

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


