Answered step by step
Verified Expert Solution
Question
1 Approved Answer
feed enthalpies A binary mixture of benzene and toluene is to be separated in a continuous distillation column at 1 atm total pressure. The mixture
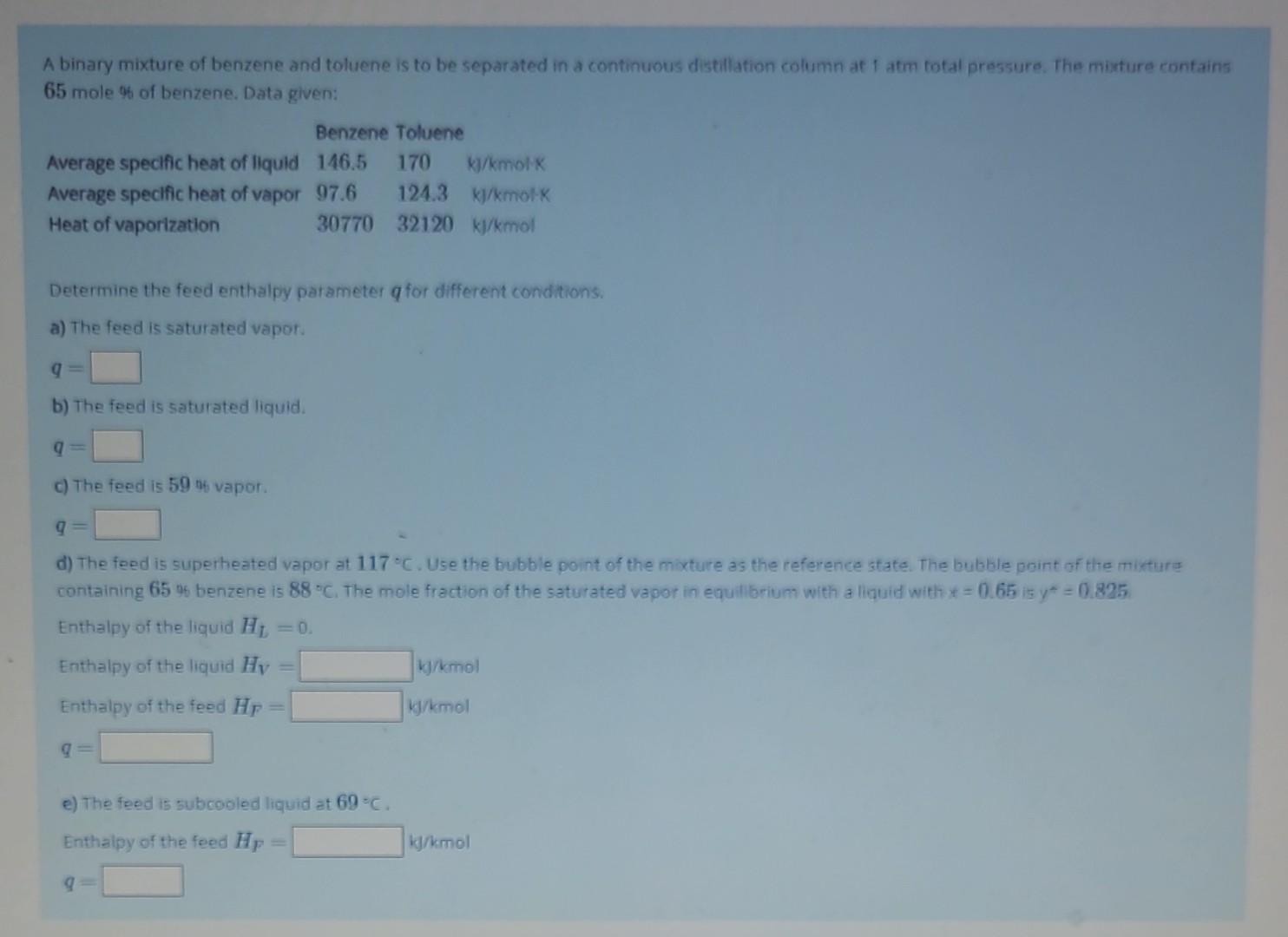
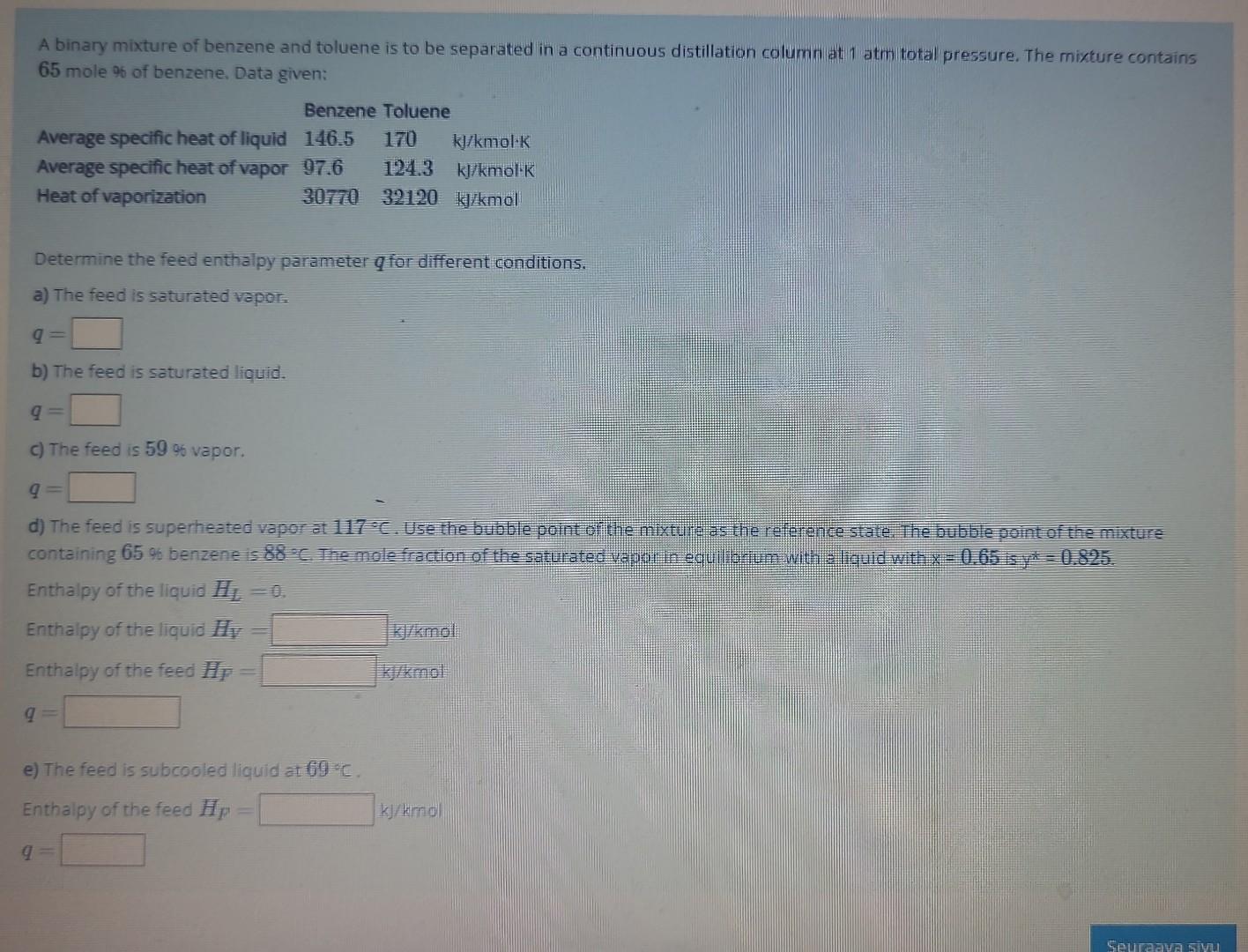
feed enthalpies
A binary mixture of benzene and toluene is to be separated in a continuous distillation column at 1 atm total pressure. The mixture contains 65 mole % of benzene, Data given: Determine the feed enthalpy parameter q for different conditions. a) The feed is saturated vapor. q= b) The feed is saturated liquid. q= c) The feed is 59 of vapor. q= d) The feed is superheated vapor at 117C. Use the bubble point of the mixture as the reference state. The bubble point of the mikture containing 65% benzene is 88C. The mole fraction of the saturated vapor in equillbrium with a liquid with x=0.65 is y=0.825 Enthalpy of the liquid HL=0. Enthalpy of the liquid HV= Enthalpy of the feed HP= q= e) The feed is subcooled liquid at 69=C. Enthalpy of the feed HF= 1k/kmol A binary mixture of benzene and toluene is to be separated in a continuous distillation column at 1 atmi total pressure. The mixture contains 65 mole % of benzene, Data given: Determine the feed enthalpy parameter q for different conditions. a) The feed is saturated vapor q= b) The feed is saturated liquid. q= c) The feed is 5996 vapor. q= d) The feed is superheated vapor at 117C. Use the bubble point of the mixtuit as the reference state. The bubble point of the mixture containing 65% benzene is 88. The mole fraction of the saturated wapar in equild ium with a licuid with x=0.65. Enthalpy of the liquid H1=0 Enthalpy of the liquid HIV= Enthalpy of the feed HF= e) The feed is subcooled liquid at 69C. Enthalpy of the feed HP=Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


