Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Few researchers studied the effects of pH on the rate of the -chymotrypsin-catalyzed hydrolysis of methyl hydrocinnamate. An initial rate approach based on potentiometric measurements
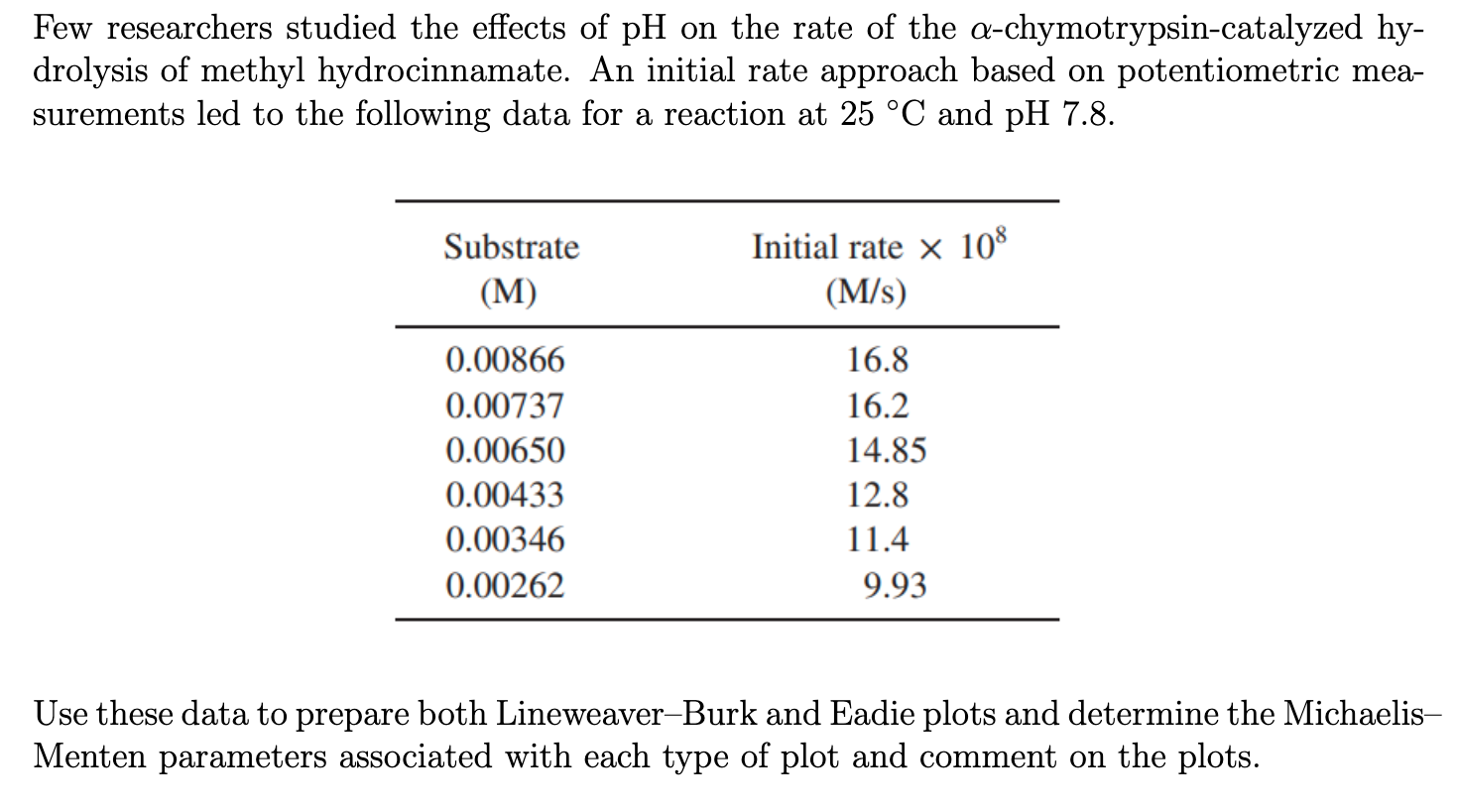 Few researchers studied the effects of pH on the rate of the -chymotrypsin-catalyzed hydrolysis of methyl hydrocinnamate. An initial rate approach based on potentiometric measurements led to the following data for a reaction at 25C and pH 7.8. Use these data to prepare both Lineweaver-Burk and Eadie plots and determine the MichaelisMenten parameters associated with each type of plot and comment on the plots. Few researchers studied the effects of pH on the rate of the -chymotrypsin-catalyzed hydrolysis of methyl hydrocinnamate. An initial rate approach based on potentiometric measurements led to the following data for a reaction at 25C and pH 7.8. Use these data to prepare both Lineweaver-Burk and Eadie plots and determine the MichaelisMenten parameters associated with each type of plot and comment on the plots
Few researchers studied the effects of pH on the rate of the -chymotrypsin-catalyzed hydrolysis of methyl hydrocinnamate. An initial rate approach based on potentiometric measurements led to the following data for a reaction at 25C and pH 7.8. Use these data to prepare both Lineweaver-Burk and Eadie plots and determine the MichaelisMenten parameters associated with each type of plot and comment on the plots. Few researchers studied the effects of pH on the rate of the -chymotrypsin-catalyzed hydrolysis of methyl hydrocinnamate. An initial rate approach based on potentiometric measurements led to the following data for a reaction at 25C and pH 7.8. Use these data to prepare both Lineweaver-Burk and Eadie plots and determine the MichaelisMenten parameters associated with each type of plot and comment on the plots Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


