Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Fifty moles per second of gas mixture analyzing 0.80 methane, 0.15 ethane, and 0.05 propane enters a burner with 0.69 fraction of excess dry air.
Fifty moles per second of gas mixture analyzing 0.80 methane, 0.15 ethane, and 0.05 propane enters a burner with 0.69 fraction of excess dry air. This furnace is connected to a boiler which produces 10 kg/s of steam at 423.15 K and a constant pressure of 1 bar using a feed of saturated boiler feed water. Use fixed-point iteration method to show that the outlet temperature of the furnace is 1035.63 K. (Additional info: feed air and fuel enter at 298.15 K).
Hint: Qnet = 23590.9 kW
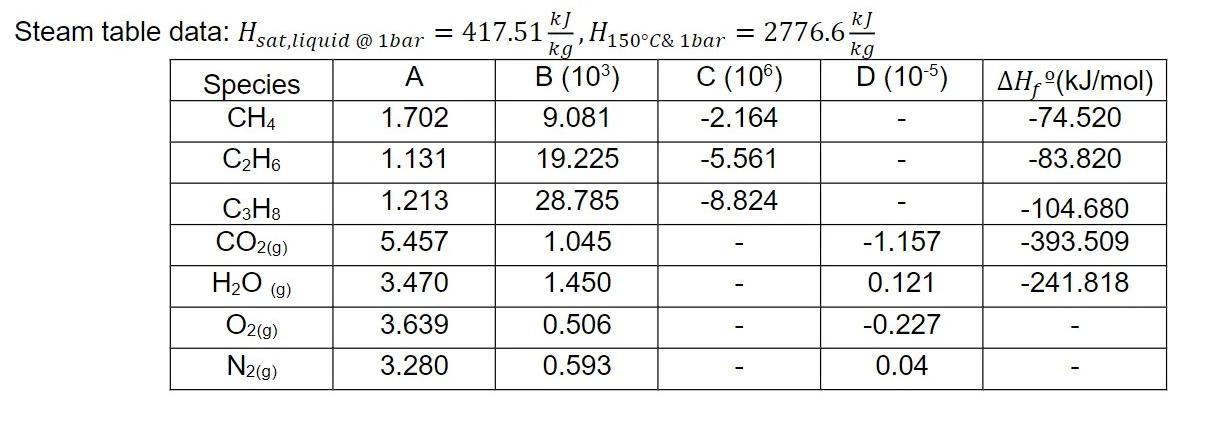
= 417.51,H150C& kg B (10) 9.081 19.225 28.785 1.045 1.450 0.506 0.593 Steam table data: Hsat,liquid @ 1bar A Species CH4 1.702 CH6 1.131 1.213 C3H8 CO2(g) 5.457 HO (g) 3.470 O2(g) 3.639 N2(g) 3.280 1bar kJ kg D (105) -1.157 0.121 -0.227 0.04 = 2776.6- C (106) -2.164 -5.561 -8.824 AH, (kJ/mol) -74.520 -83.820 -104.680 -393.509 -241.818
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


