Question
Figure 1 shows the phase diagram of platinum-silver system. 1. To what type of phase diagrams figure 1 refers to? binary peritectic platinum-silver system 2.
Figure 1 shows the phase diagram of platinum-silver system. 1. To what type of phase diagrams figure 1 refers to? binary peritectic platinum-silver system 2. What the lines Line L, Line M and Line N are called? liquidus, solidus and solvus 3. Consider an alloy with nominal composition of 30 wt% Ag being cooled from a temperature of 1400C. At the instant when the temperature reaches 1150C, the phase (or phases) present will most probably be .................. solid + solid 4. Calculate the weight fraction of each ph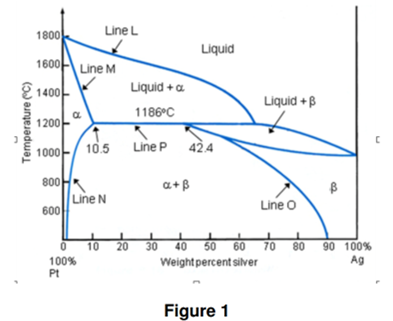 ase, which will form at 1180C in Pt-20wt% Ag alloy. : 0.7 and : 0.3
ase, which will form at 1180C in Pt-20wt% Ag alloy. : 0.7 and : 0.3
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


