Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Figure 1 shows the phase diagram of the gold-germanium (Au-Ge) binary cutectic sys- tem. Answer the following questions according to the phase diagram. Atomic Percent
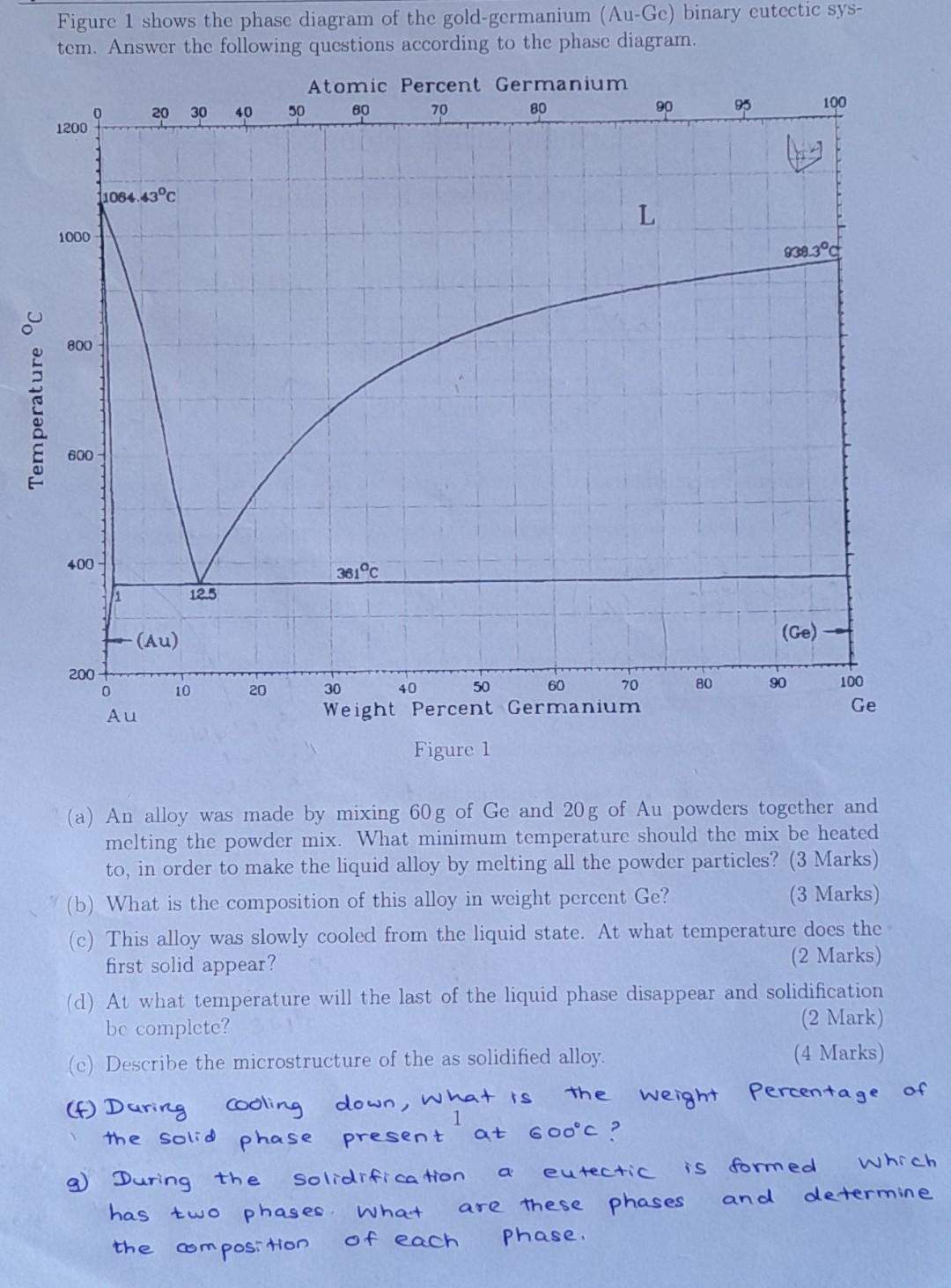
Figure 1 shows the phase diagram of the gold-germanium (Au-Ge) binary cutectic sys- tem. Answer the following questions according to the phase diagram. Atomic Percent Germanium 50 B0 70 80 20 30 100 40 90 1200 1064.43C L 1000 839.3C 800 Temperature C 600 400 381C 125 (Au) (Ge) 200 0 30 10 40 20 50 80 60 90 70 100 Ge Au Weight Percent Germanium Figure 1 (a) An alloy was made by mixing 60 g of Ge and 20g of Au powders together and melting the powder mix. What minimum temperature should the mix be heated to, in order to make the liquid alloy by melting all the powder particles? (3 Marks) (b) What is the composition of this alloy in weight percent Ge? (3 Marks) (c) This alloy was slowly cooled from the liquid state. At what temperature does the first solid appear? (2 Marks) (d) At what temperature will the last of the liquid phase disappear and solidification be complete? (2 Mark) (c) Describe the microstructure of the as solidified alloy (4 Marks) (f) During cooling down, what is weight percentage of 1 the solid phase present at Gooc? Solidification g) During the eutectic Which is formed has two phases. What are these phases and determine the of each compositor phase. the a
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


