Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Figure Q1 below shows the equilibrium phase diagram of Lead (Pb)-Tin (Sn) alloy system. (a) (b) (c) Temperature (C) (ii) 300 200 100 327C
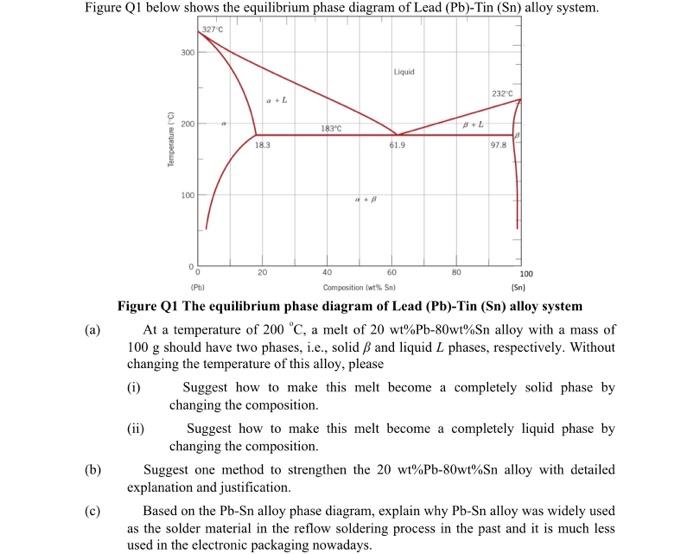
Figure Q1 below shows the equilibrium phase diagram of Lead (Pb)-Tin (Sn) alloy system. (a) (b) (c) Temperature (C) (ii) 300 200 100 327C a+L 18.3 20 183C 40 *** Liquid 61.9 60 (Pb) Composition (wt% Sn) (Sn) Figure Q1 The equilibrium phase diagram of Lead (Pb)-Tin (Sn) alloy system At a temperature of 200 C, a melt of 20 wt% Pb-80wt% Sn alloy with a mass of 100 g should have two phases, i.e., solid and liquid L phases, respectively. Without changing the temperature of this alloy, please (i) 80 232 C 97.8 100 Suggest how to make this melt become a completely solid phase by changing the composition. Suggest how to make this melt become a completely liquid phase by changing the composition. Suggest one method to strengthen the 20 wt% Pb-80wt% Sn alloy with detailed explanation and justification. Based on the Pb-Sn alloy phase diagram, explain why Pb-Sn alloy was widely used as the solder material in the reflow soldering process in the past and it is much less used in the electronic packaging nowadays.
Step by Step Solution
★★★★★
3.45 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
Question 350 300 250 200 150 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


