Question
Fill in the blanks Half Reaction F(g) + 2e 2F (aq) Cl(g) + 2e 2Cl(aq) Br(g) + 2e 2Br (aq) O(g) + 4H*(aq) +
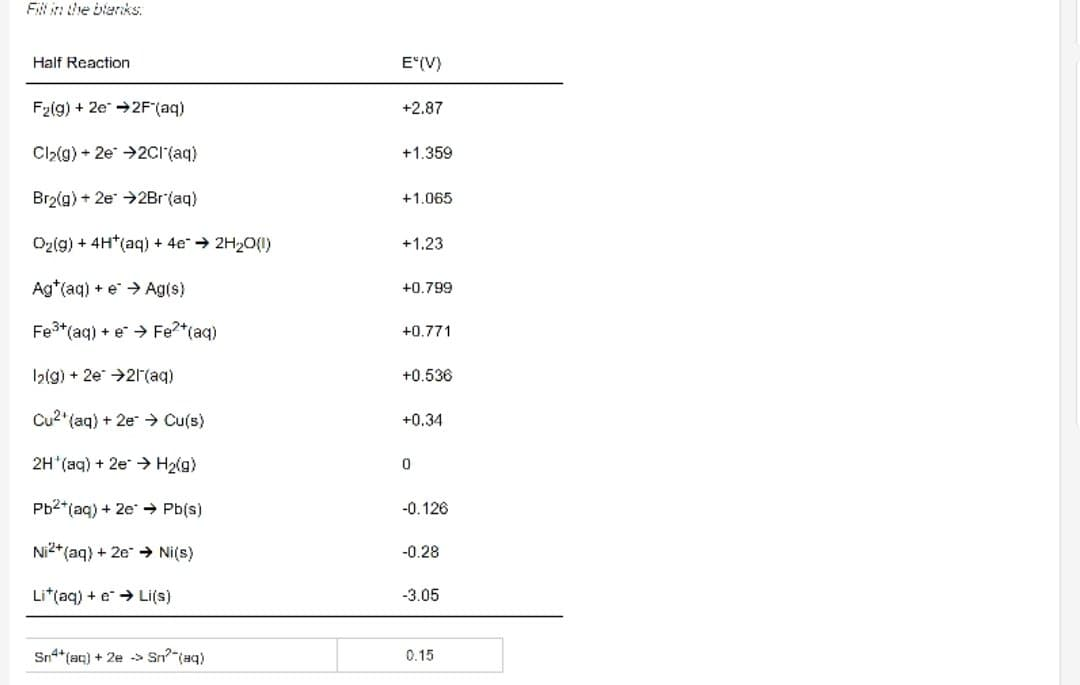
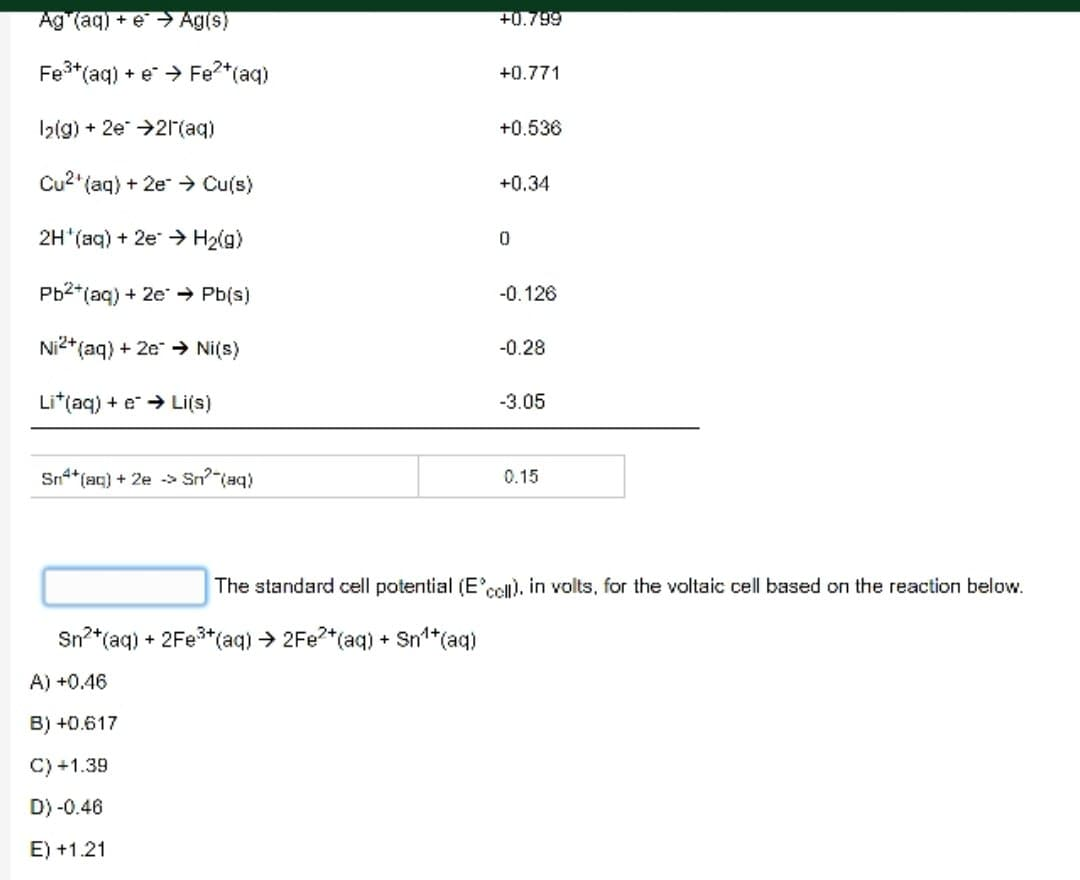
Fill in the blanks Half Reaction F(g) + 2e 2F (aq) Cl(g) + 2e 2Cl(aq) Br(g) + 2e 2Br (aq) O(g) + 4H*(aq) + 4e 2HO(l) Ag (aq) + e Ag(s) Fe+ (aq) + e Fe+ (aq) 1(g) + 2e 21(aq) Cu2 (aq) + 2e Cu(s) 2H (aq) + 2e H(g) Pb2+ (aq) + 2e Pb(s) Ni2+ (aq) + 2e Ni(s) Lit(aq) + e Li(s) Sn4+ (aq) + 2e > Sn? (aq) E(V) +2.87 +1.359 +1.065 +1.23 +0.799 +0.771 +0.536 +0.34 0 -0.126 -0.28 -3.05 0.15 Ag (aq) + e Ag(s) Fe+ (aq) + e* Fe+ (aq) 1(g) + 2e 21(aq) Cu2 (aq) + 2e Cu(s) 2H(aq) + 2e H(g) Pb+ (aq) + 2e Pb(s) Ni2+ (aq) + 2e Ni(s) Lit(aq) + e Li(s) Sn+ (aq) + 2e > Sn (aq) +0.799 Sn+ (aq) + 2Fe+ (aq) 2Fe2+ (aq) + Sn+ (aq) A) +0.46 B) +0.617 C) +1.39 D) -0.46 E) +1.21 +0.771 +0.536 +0.34 0 -0.126 -0.28 -3.05 0.15 The standard cell potential (E'cell). in volts, for the voltaic cell based on the reaction below.
Step by Step Solution
3.41 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Cambridge International AS And A Level Chemistry Coursebook
Authors: Lawrie Ryan, Roger Norris
2nd Edition
1316637735, 978-1316637739
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



