Question
Find the molarity of sample 1 for trial one and sample 2 trial one. Trial one Trial two Trial three Volume Sample 1 Starting volume
Find the molarity of sample 1 for trial one and sample 2 trial one.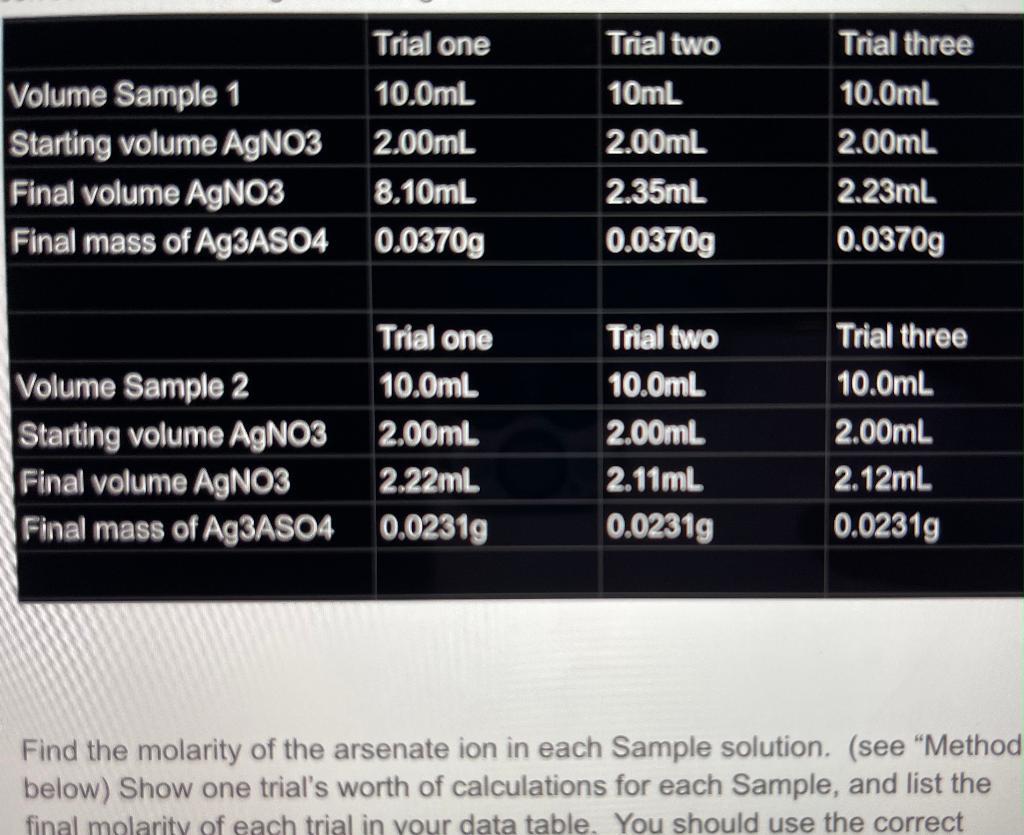
Trial one Trial two Trial three Volume Sample 1 Starting volume AGNO3 2.00mL Final volume AGNO3 Final mass of A93ASO4 0.0370g 10.0mL 10mL 10.0mL 2.00mL 2.00mL 8.10mL 2.35mL 2.23mL 0.0370g 0.0370g Trial one Trial two Trial three Volume Sample 2 Starting volume AgNO3 Final volume AGNO3 Final mass of Ag3ASO4 0.0231g 10.0mL 10.0mL 10.0mL 2.00mL 2.00mL 2.00mL 2.22mL 2.11mL 2.12mL 0.0231g 0.0231g Find the molarity of the arsenate ion in each Sample solution. (see "Method below) Show one trial's worth of calculations for each Sample, and list the final molarity of each trial in your data table. You should use the correct
Step by Step Solution
3.45 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Differential Equations and Linear Algebra
Authors: Jerry Farlow, James E. Hall, Jean Marie McDill, Beverly H. West
2nd edition
131860615, 978-0131860612
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



