Question: Find the equilibrium concentration for each MISSED THIS? Read Section 16.8 (Pages 701710); Watch KCV 16.8, IWE 16.9. Consider the reaction What will be the
Find the equilibrium concentration for each
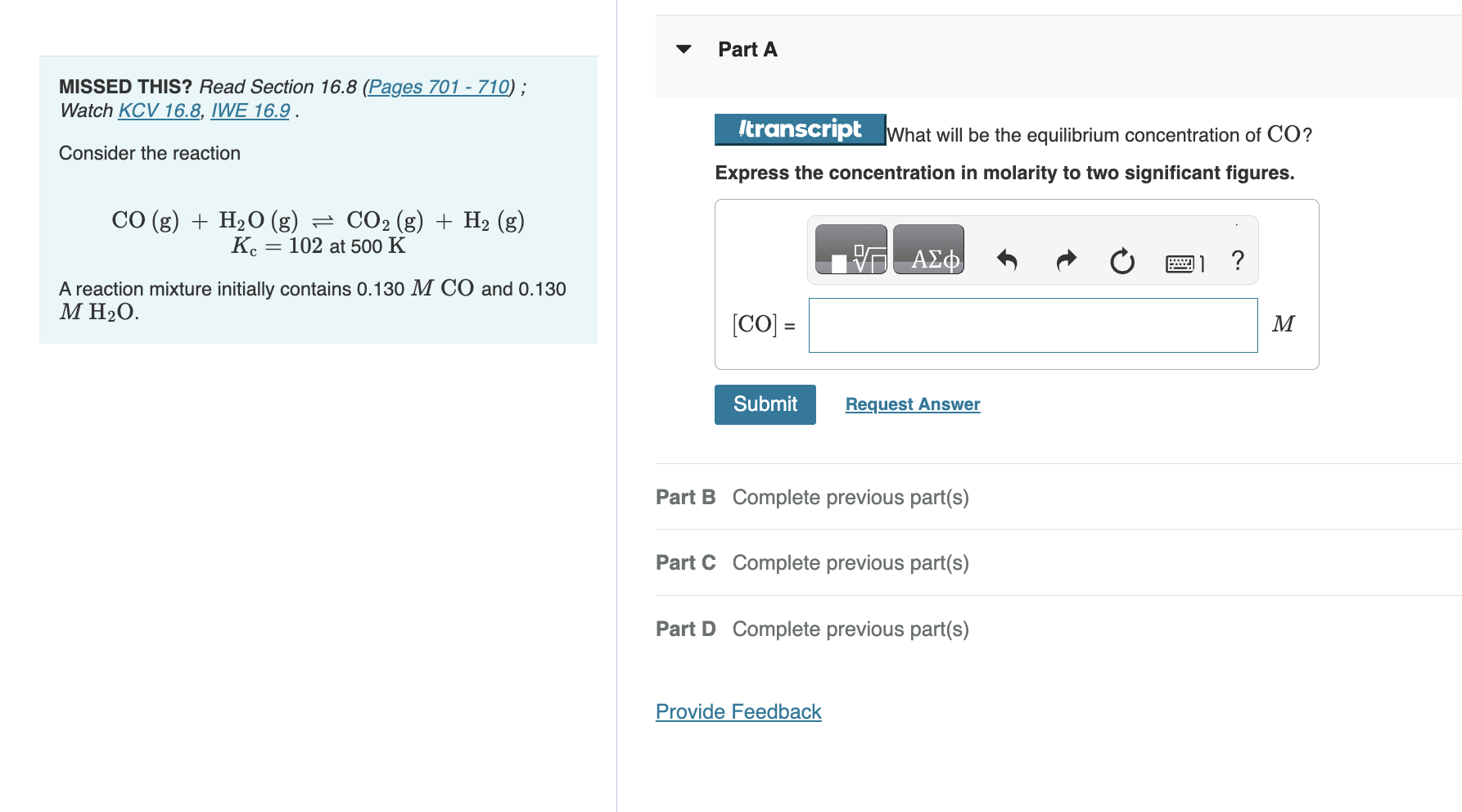
MISSED THIS? Read Section 16.8 (Pages 701710); Watch KCV 16.8, IWE 16.9. Consider the reaction What will be the equilibrium concentration of CO ? Express the concentration in molarity to two significant figures. CO(g)+H2O(g)CO2(g)+H2(g)Kc=102at500K A reaction mixture initially contains 0.130MCO and 0.130 MH2O. [CO]= M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


