Answered step by step
Verified Expert Solution
Question
1 Approved Answer
find the mass of NH3 as well Find the mass of NH3 as well Himitiof eeortant: Masis of CO e Limiting rebctant Hask af NO=
find the mass of NH3 as well 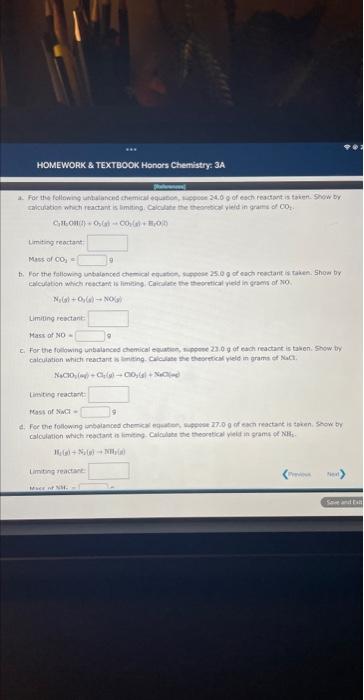

Find the mass of NH3 as well 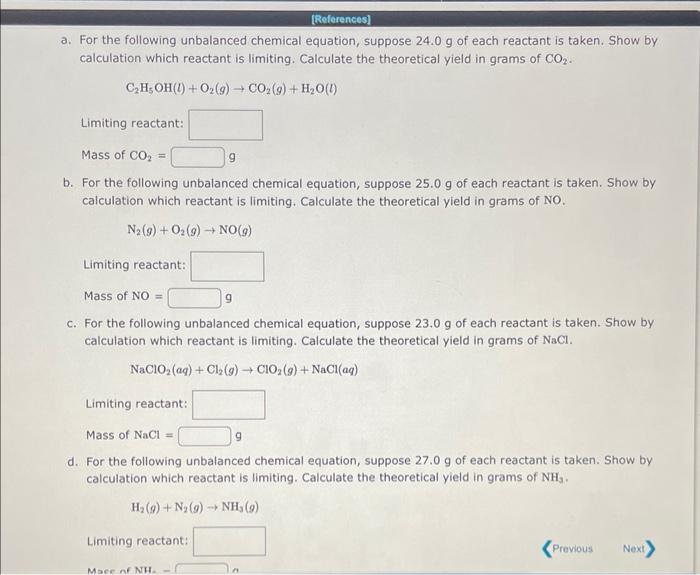
Himitiof eeortant: Masis of CO e Limiting rebctant Hask af NO= C. For the follwing. unbalanced chemical equatien, s pesce 22.9g of esch feadant is taken: Show ty calcuation which reaitarit is armeing. Calculase the theorefica yield in grams et NaCt. Lintiving reactarh thas of anth = If1(e)+N(e)=NT/B) iimting reactant. a. For the following unbalanced chemical equation, suppose 24.0g of each reactant is taken. Show by calculation which reactant is limiting. Calculate the theoretical yield in grams of CO2. C2H5OH(l)+O2(g)CO2(g)+H2O(l) Limiting reactant: Mass of CO2= 9 b. For the following unbalanced chemical equation, suppose 25.0g of each reactant is taken. Show by calculation which reactant is limiting. Calculate the theoretical yield in grams of NO. N2(g)+O2(g)NO(g) Limiting reactant: MassofNO=9 c. For the following unbalanced chemical equation, suppose 23.0g of each reactant is taken. Show by calculation which reactant is limiting. Calculate the theoretical yield in grams of NaCl. NaClO2(aq)+Cl2(g)ClO2(g)+NaCl(aq) Limiting reactant: Mass of NaCl= 9 d. For the following unbalanced chemical equation, suppose 27.0g of each reactant is taken. Show by calculation which reactant is limiting. Calculate the theoretical yield in grams of NH3. H2(g)+N2(g)NH3(g) Limiting reactant 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


