Question
Find the residual enthalpy for a mole of ethane gas at a temperature of 300K and a pressure of 2 bar, that follows the following
Find the residual enthalpy for a mole of ethane gas at a temperature of 300K and a pressure of 2 bar, that follows the following equation of state (a variation of the Redlich-Kwong EOS):
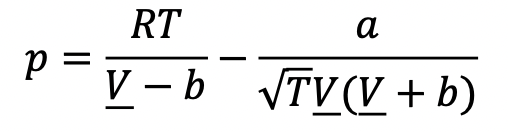 where:
where:
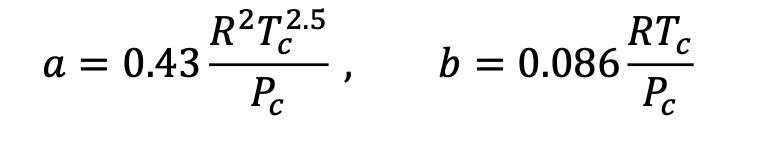
In order to solve this, you will need to find the Z value at the conditions for the residual (T = 300K and pressure of 2 bar) but because this is difficult with a cubic equation of state (to find volume) I will tell you that the volume at this temperature and pressure (in its gas phase and therefore the largest of the three volumes) is 0.013 m3/mol. Convert your residual enthalpy value into J/mol, and comment whether it seems if residuals, and all this work, are important for this condition.
p=VbRTTV(V+b)a a=0.43PcR2Tc2.5,b=0.086PcRTcStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


