Answered step by step
Verified Expert Solution
Question
1 Approved Answer
first picture shows question! i am so confused! pleaee help ASAP! the rest of the photos are all the additional experiment info i have Report
first picture shows question! i am so confused! pleaee help ASAP!
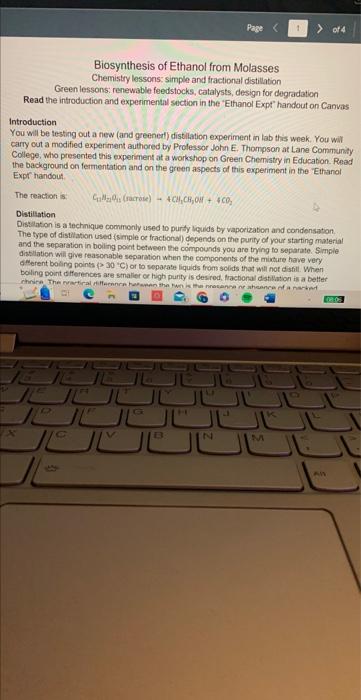
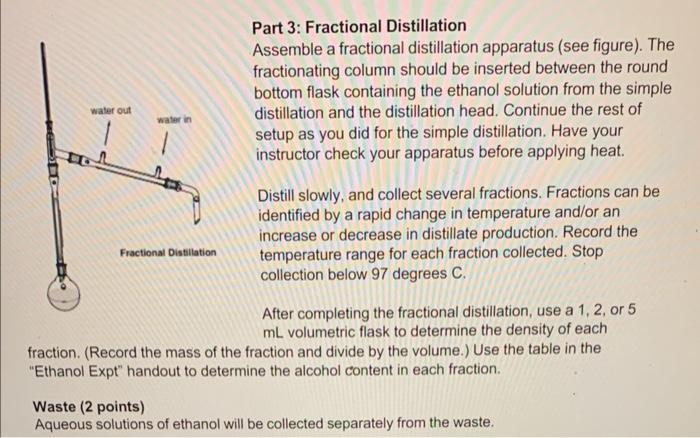
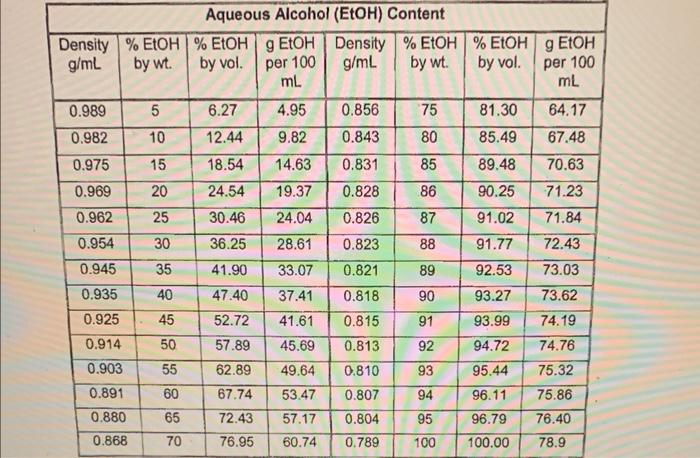
Report your results including: a. (2 points) volume collected and % ethanol for each fraction. 1. Volume collected- 0.804g/mL%96.79% 2. Volume collected 0.836g/mL%89.48% 3. Volume collected 0.962g/ml%30.46% b. (2 points) \% yield of ethanol from molasses 1. 2. 3. c. ( 2 points) \% yield ethanol from sugars, assuming approximately half of molasses is reactive sugars Biosynthesis of Ethanol from Molasses Chemistry lessons: simple and fractional distillation Green lessons: renewable feedstocks, catalysts, design for degradation Read the introduction and experimental section in the 'Elhanol Expt' handout on Canvas Introduction You will be testing out a new (and greenerl) distilation experiment in lab this week. You wilf carry out a modified experiment authored by Prolessor John E. Thompsan at Lane Community College, who presented this experinent at a workshop on Green Chemistry in Education. Read the background on fermentation and on the green aspects of this experiment in the Ethanol Expr handout. The reaction is: CaH2Ois( (raquase) 4CH2CH3 our +4CO2 Distillation Dist:lation is a lechnique cominonly used to pursy liguds by vaporizabion and condensation. The type of distlation used (simple ce fractional) depends on the punty of your starting material and the separation in boling point between the compounds you are tring to separate. Simple ditstiation wili give reasonable separation when the components of the mixture have very a Merent boing poins (230 C) or to sepacase liquids froms solids that ill not disali. When. boling point diferences are smaller or high purty is desred, fracbonal distlitition is a better Part 1: Set up and fermentation Mix 50mL molasses with 50mL distilled water in a 250mL filter flask. Add about 0.5g yeast and stir gently until well mixed. Add a rubber stopper to the top of the filter flask. Attach a short rubber hose to the sidearm of the filter flask, and insert a short straight section of glass tube into the other end of the rubber tube. Dip the straight glass tube into a large test tube two-thirds full of Ca(OH)2 solution (limewater). This creates a one-way vapor lock that will inhibit air from entering the flask while allowing carbon dioxide to escape. (The presence of air would lead to overoxidation of ethanol to acetic acid.) Store the reaction in your drawer until the next lab period. Part 2: Simple Distillation Decant about 50mL of the ethanol solution into a 100mL round-bottomed flask. Assemble the remaining glassware for simple distillation (see figure below), including a distillation head, distillation column (condenser), bent adapter, and water hosing (Note: water enters on the bottom and leaves out of the top). Only a gentle trickle is required! Do not turn water on too high! Add a thermometer and thermometer adapter. Position the thermometer so that the bulb is just below the level of the side arm. Lower the round-bottomed flask into a heating mantle. Have Distill at a rate of about 1 drop per second. Soon after boiling begins, a ring of condensing vapor will be seen rising in the flask and neck. Once steady distillation is underway, the thermometer bulb should be bathed in vapor and have a constant drop of condensate on the tip. In this condition, it will register the temperature at which liquid and vapor are in equilibrium, i.e. the boiling point. The temperature in the distilling head will not level off to the boiling point until thermal equilibrium with the glass walls is established: this requires a minute or two, and at least a drop of distillate will be collected before the boiling point is registered. The heating rate should be adjusted to maintain a distillation rate of about one drop per second. If heating is too rapid, an appreciable amount of distillate may appear to be lower-boiling. If heating is too slow, the true boiling point may never be observed because of heat losses. Collect the alcohol fraction until just below the boiling point of water. Safety: Do NOT distill to dryness! Distillation must always be stopped before the flask becomes completely dry. Without the absorption of heat due to vaporization, the temperature can rise extremely rapidly, sometimes sufficient to crack the vessel. Also, many liquids - particularly alkenes and ethers - may contain peroxides that become concentrated into highly explosive residues. Part 3: Fractional Distillation Assemble a fractional distillation apparatus (see figure). The fractionating column should be inserted between the round bottom flask containing the ethanol solution from the simple distillation and the distillation head. Continue the rest of setup as you did for the simple distillation. Have your instructor check your apparatus before applying heat. Distill slowly, and collect several fractions. Fractions can be identified by a rapid change in temperature and/or an increase or decrease in distillate production. Record the temperature range for each fraction collected. Stop collection below 97 degrees C. After completing the fractional distillation, use a 1, 2 , or 5 mL volumetric flask to determine the density of each fraction. (Record the mass of the fraction and divide by the volume.) Use the table in the "Ethanol Expt" handout to determine the alcohol content in each fraction. Waste (2 points) Aqueous solutions of ethanol will be collected separately from the waste. Molasses is a mixture of monosaccharides and disaccharides and other miscellaneous flavoring agents naturally produced in the sugar cane or sugar beet. Approximately 50% of the molasses mass is sugars. Yeast has two enzymes that convert the saccharides to ethanol. Invertase converts dissacharides (such as sucrose) to monosaccharides by a catalytic hydrolysis (addition of water) reaction. Glucose and fructose are then converted to ethanol and carbon dioxide by the enzyme zymase. One mole of sucrose will produce four moles of ethanol and four moles of carbon dioxide. When the alcohol level becomes high enough the yeast will die of alcohol poisoning. The reaction is vapor locked with limewater so that the reaction environment remains oxygen free keeping the ethanol from further oxidizing to acetic acid. Boiling occurs when the vapor pressure of a liquid is equal to the atmospheric pressure. This is determined using Raoult's law for an ideal gas. Distillations take advantage of this behavior in a mix of solutions to separate two liquids. Some liquids do not behave in this ideal way and instead form azeotropes. Ethanol and water form a temperature minimum azeotrope, making pure ethanol very difficult to obtain. This azeotrope boils at 78.2C with a 95.6% ethanol, 4.4% water blend. To better understand this process read the distillation theory section of the laboratory techniques manual. Amuanue MIankal IEthHI Confant Report your results including: a. (2 points) volume collected and % ethanol for each fraction. 1. Volume collected- 0.804g/mL%96.79% 2. Volume collected 0.836g/mL%89.48% 3. Volume collected 0.962g/ml%30.46% b. (2 points) \% yield of ethanol from molasses 1. 2. 3. c. ( 2 points) \% yield ethanol from sugars, assuming approximately half of molasses is reactive sugars Biosynthesis of Ethanol from Molasses Chemistry lessons: simple and fractional distillation Green lessons: renewable feedstocks, catalysts, design for degradation Read the introduction and experimental section in the 'Elhanol Expt' handout on Canvas Introduction You will be testing out a new (and greenerl) distilation experiment in lab this week. You wilf carry out a modified experiment authored by Prolessor John E. Thompsan at Lane Community College, who presented this experinent at a workshop on Green Chemistry in Education. Read the background on fermentation and on the green aspects of this experiment in the Ethanol Expr handout. The reaction is: CaH2Ois( (raquase) 4CH2CH3 our +4CO2 Distillation Dist:lation is a lechnique cominonly used to pursy liguds by vaporizabion and condensation. The type of distlation used (simple ce fractional) depends on the punty of your starting material and the separation in boling point between the compounds you are tring to separate. Simple ditstiation wili give reasonable separation when the components of the mixture have very a Merent boing poins (230 C) or to sepacase liquids froms solids that ill not disali. When. boling point diferences are smaller or high purty is desred, fracbonal distlitition is a better Part 1: Set up and fermentation Mix 50mL molasses with 50mL distilled water in a 250mL filter flask. Add about 0.5g yeast and stir gently until well mixed. Add a rubber stopper to the top of the filter flask. Attach a short rubber hose to the sidearm of the filter flask, and insert a short straight section of glass tube into the other end of the rubber tube. Dip the straight glass tube into a large test tube two-thirds full of Ca(OH)2 solution (limewater). This creates a one-way vapor lock that will inhibit air from entering the flask while allowing carbon dioxide to escape. (The presence of air would lead to overoxidation of ethanol to acetic acid.) Store the reaction in your drawer until the next lab period. Part 2: Simple Distillation Decant about 50mL of the ethanol solution into a 100mL round-bottomed flask. Assemble the remaining glassware for simple distillation (see figure below), including a distillation head, distillation column (condenser), bent adapter, and water hosing (Note: water enters on the bottom and leaves out of the top). Only a gentle trickle is required! Do not turn water on too high! Add a thermometer and thermometer adapter. Position the thermometer so that the bulb is just below the level of the side arm. Lower the round-bottomed flask into a heating mantle. Have Distill at a rate of about 1 drop per second. Soon after boiling begins, a ring of condensing vapor will be seen rising in the flask and neck. Once steady distillation is underway, the thermometer bulb should be bathed in vapor and have a constant drop of condensate on the tip. In this condition, it will register the temperature at which liquid and vapor are in equilibrium, i.e. the boiling point. The temperature in the distilling head will not level off to the boiling point until thermal equilibrium with the glass walls is established: this requires a minute or two, and at least a drop of distillate will be collected before the boiling point is registered. The heating rate should be adjusted to maintain a distillation rate of about one drop per second. If heating is too rapid, an appreciable amount of distillate may appear to be lower-boiling. If heating is too slow, the true boiling point may never be observed because of heat losses. Collect the alcohol fraction until just below the boiling point of water. Safety: Do NOT distill to dryness! Distillation must always be stopped before the flask becomes completely dry. Without the absorption of heat due to vaporization, the temperature can rise extremely rapidly, sometimes sufficient to crack the vessel. Also, many liquids - particularly alkenes and ethers - may contain peroxides that become concentrated into highly explosive residues. Part 3: Fractional Distillation Assemble a fractional distillation apparatus (see figure). The fractionating column should be inserted between the round bottom flask containing the ethanol solution from the simple distillation and the distillation head. Continue the rest of setup as you did for the simple distillation. Have your instructor check your apparatus before applying heat. Distill slowly, and collect several fractions. Fractions can be identified by a rapid change in temperature and/or an increase or decrease in distillate production. Record the temperature range for each fraction collected. Stop collection below 97 degrees C. After completing the fractional distillation, use a 1, 2 , or 5 mL volumetric flask to determine the density of each fraction. (Record the mass of the fraction and divide by the volume.) Use the table in the "Ethanol Expt" handout to determine the alcohol content in each fraction. Waste (2 points) Aqueous solutions of ethanol will be collected separately from the waste. Molasses is a mixture of monosaccharides and disaccharides and other miscellaneous flavoring agents naturally produced in the sugar cane or sugar beet. Approximately 50% of the molasses mass is sugars. Yeast has two enzymes that convert the saccharides to ethanol. Invertase converts dissacharides (such as sucrose) to monosaccharides by a catalytic hydrolysis (addition of water) reaction. Glucose and fructose are then converted to ethanol and carbon dioxide by the enzyme zymase. One mole of sucrose will produce four moles of ethanol and four moles of carbon dioxide. When the alcohol level becomes high enough the yeast will die of alcohol poisoning. The reaction is vapor locked with limewater so that the reaction environment remains oxygen free keeping the ethanol from further oxidizing to acetic acid. Boiling occurs when the vapor pressure of a liquid is equal to the atmospheric pressure. This is determined using Raoult's law for an ideal gas. Distillations take advantage of this behavior in a mix of solutions to separate two liquids. Some liquids do not behave in this ideal way and instead form azeotropes. Ethanol and water form a temperature minimum azeotrope, making pure ethanol very difficult to obtain. This azeotrope boils at 78.2C with a 95.6% ethanol, 4.4% water blend. To better understand this process read the distillation theory section of the laboratory techniques manual. Amuanue MIankal IEthHI Confant the rest of the photos are all the additional experiment info i have 










Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


