Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Following Bohr, we assume that a hydrogen-like atom may be modelled as a single electron (mass m and charge -e) in a circular orbit
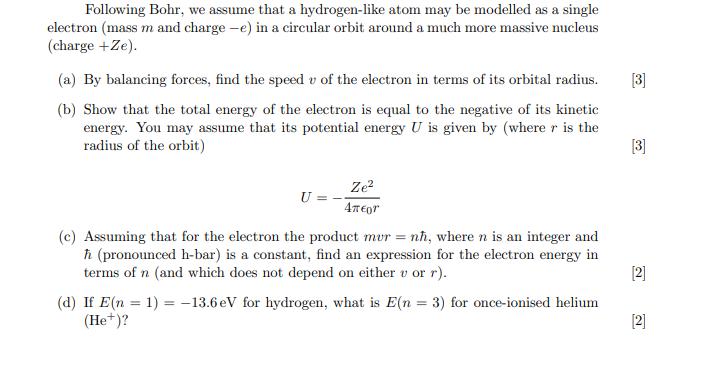
Following Bohr, we assume that a hydrogen-like atom may be modelled as a single electron (mass m and charge -e) in a circular orbit around a much more massive nucleus (charge +Ze). (a) By balancing forces, find the speed v of the electron in terms of its orbital radius. (b) Show that the total energy of the electron is equal to the negative of its kinetic energy. You may assume that its potential energy U is given by (where r is the radius of the orbit) (d) If E(n (He+)? (c) Assuming that for the electron the product mur = n, where n is an integer and (pronounced h-bar) is a constant, find an expression for the electron energy in terms of n (and which does not depend on either v or r). 13.6 eV for hydrogen, what is E(n = 3) for once-ionised helium = U= 1) Ze 4or [3] [3] [2] [2]
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The Bohr model of a hydrogenlike atom considers a single electron with mass m and charge e in orbit ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


