Question
For a first order reaction, the initial reactant concentration, [A]o, is 0.76 M. After 17.7 s, the concentration is 0.65 M. What is [A]
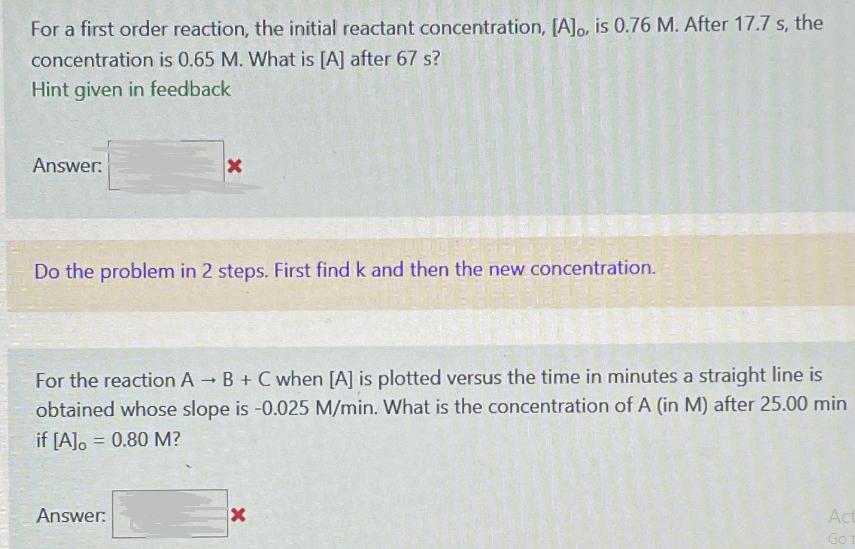
For a first order reaction, the initial reactant concentration, [A]o, is 0.76 M. After 17.7 s, the concentration is 0.65 M. What is [A] after 67 s? Hint given in feedback Answer: X Do the problem in 2 steps. First find k and then the new concentration. For the reaction A B + C when [A] is plotted versus the time in minutes a straight line is obtained whose slope is -0.025 M/min. What is the concentration of A (in M) after 25.00 min if [A] = 0.80 M? Answer: x Act Go 1
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The image youve provided contains two chemistry questions related to reaction kinetics for firstorde...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



