Answered step by step
Verified Expert Solution
Question
1 Approved Answer
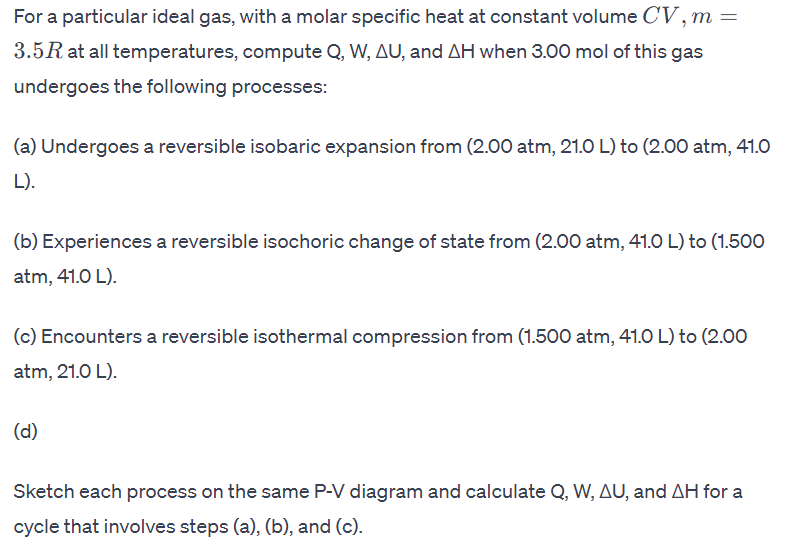
For a particular ideal gas, with a molar specific heat at constant volume C V , m = 3 . 5 R at all temperatures,
For a particular ideal gas, with a molar specific heat at constant volume
at all temperatures, compute and when mol of this gas
undergoes the following processes:
a Undergoes a reversible isobaric expansion from atm, to
L
b Experiences a reversible isochoric change of state from atm, to
atm,
c Encounters a reversible isothermal compression from atm, to
atm, L
d
Sketch each process on the same diagram and calculate and for a
cycle that involves steps ab and c
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


