Question
For each of the following molecules, (i) write the valence electron configuration (Your answer should be in a fom similar to (o2s), which is
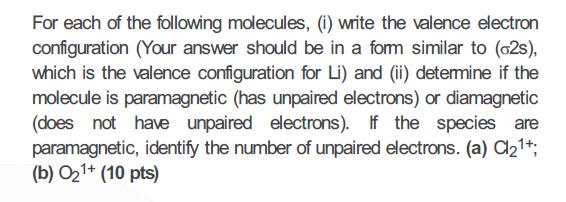
For each of the following molecules, (i) write the valence electron configuration (Your answer should be in a fom similar to (o2s), which is the valence configuration for Li) and (ii) detemine if the molecule is paramagnetic (has unpaired electrons) or diamagnetic (does not hae unpaired electrons). If the species are paramagnetic, identify the number of unpaired electrons. (a) Cl21*; (b) O21+ (10 pts)
Step by Step Solution
3.39 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Quantum Chemistry
Authors: Ira N. Levine
7th edition
321803450, 978-0321803450
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



