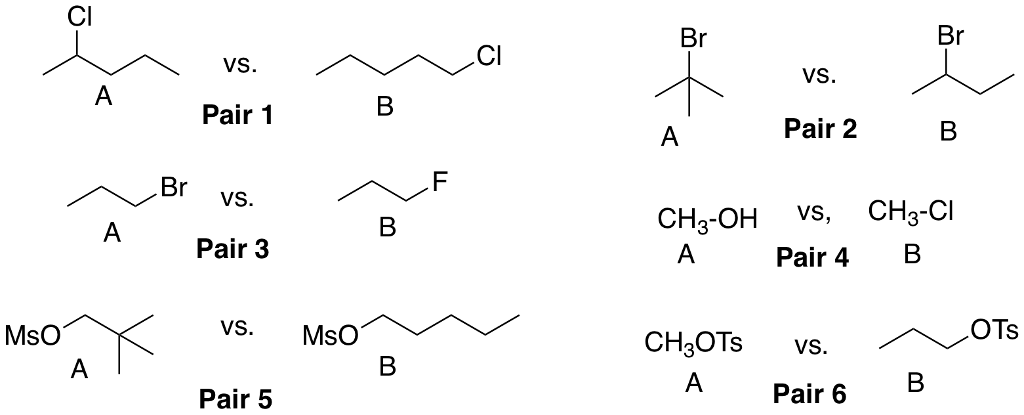
Question: For each of the given pairs indicate which substance (A or B) will react faster in an SN2 reaction by selecting the correct compound from
For each of the given pairs indicate which substance (A or B) will react faster in an SN2 reaction by selecting the correct compound from the dropdown menu. Then select the chemical principle why that substance has a faster SN2 reaction.
Comparing Pair 1? : Compound [A,B,Both Equal] has a faster SN2 reaction due to ["a better leaving group", "a worse leaving group", "more branches at the reacting carbon", "fewer branches at the reacting carbon", "more steric hindrance near the leaving group", "less steric hindrance near the leaving group", "some other reason"]
Comparing Pair 2? : Compound [A,B,Both Equal] has a faster SN2 reaction due to ["a better leaving group", "a worse leaving group", "more branches at the reacting carbon", "fewer branches at the reacting carbon", "more steric hindrance near the leaving group", "less steric hindrance near the leaving group", "some other reason"]
Comparing Pair 3? : Compound [A,B,Both Equal] has a faster SN2 reaction due to ["a better leaving group", "a worse leaving group", "more branches at the reacting carbon", "fewer branches at the reacting carbon", "more steric hindrance near the leaving group", "less steric hindrance near the leaving group", "some other reason"]
Comparing Pair 4? : Compound [A,B,Both Equal] has a faster SN2 reaction due to ["a better leaving group", "a worse leaving group", "more branches at the reacting carbon", "fewer branches at the reacting carbon", "more steric hindrance near the leaving group", "less steric hindrance near the leaving group", "some other reason"]
Comparing Pair 5? : Compound [A,B,Both Equal] has a faster SN2 reaction due to ["a better leaving group", "a worse leaving group", "more branches at the reacting carbon", "fewer branches at the reacting carbon", "more steric hindrance near the leaving group", "less steric hindrance near the leaving group", "some other reason"]
Comparing Pair 6? : Compound [A,B,Both Equal] has a faster SN2 reaction due to ["a better leaving group", "a worse leaving group", "more branches at the reacting carbon", "fewer branches at the reacting carbon", "more steric hindrance near the leaving group", "less steric hindrance near the leaving group", "some other reason"]
MsO J- A A A Br VS. Pair 1 VS. Pair 3 VS. Pair 5 MsO B B B F Br t VS. CH3OTs A Pair 2 CH3-OH VS, A Pair 4 VS. Pair 6 Br B B CH3-CI B OTS
Step by Step Solution
3.46 Rating (153 Votes )
There are 3 Steps involved in it
To determine which compounds react faster in an SN2 reaction lets consider some key principles 1 Ste... View full answer

Get step-by-step solutions from verified subject matter experts


