Question
For each of the two water samples below, (a) and (b), calculate (pts per water sample): meq/L required chemical addition of soda ash (Na2CO3) to
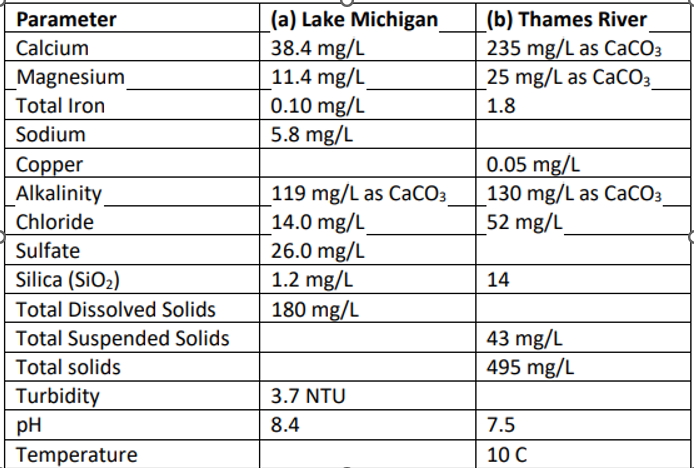
For each of the two water samples below, (a) and (b), calculate (pts per water sample):
meq/L required chemical addition of soda ash (Na2CO3) to achieve softening
kg/L of total solids produced in the softening process, considering that 7 mg/L of suspended solids is also removed through coagulation in the lime-soda softening process
actual chemical additions if lime is 88% pure and soda ash is 98% pure
the annual cost to treat 0.5 m3 /s (~11.5 MGD) of water if lime is $61.70 per megagram (Mg) and soda ash is $172.50 per megagram (Mg)
meq/L of CO2 added to water to convert half of the 0.6 meq/L of carbonate in the finished water to bicarbonate and to react with the 1.45 meq/L of OH- ions in the finished water to avoid pipe corrosion
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


