Answered step by step
Verified Expert Solution
Question
1 Approved Answer
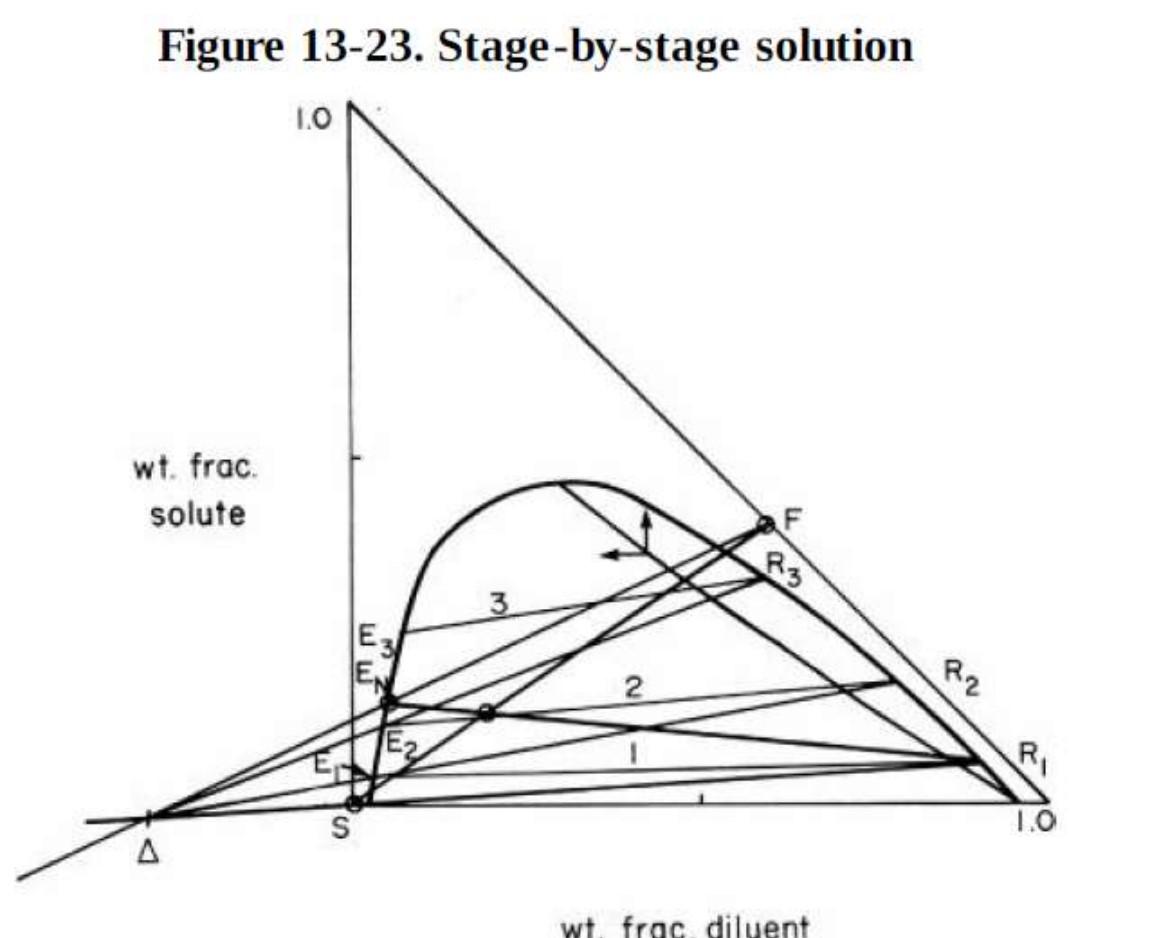
For Figure 13-23 suppose we desired to obtain the desired raffinate concentration with exactly two equilibrium stages. This can be accomplished by changing the amount
For Figure 13-23 suppose we desired to obtain the desired raffinate concentration with exactly two equilibrium stages. This can be accomplished by changing the amount of solvent used. Would we want to increase or decrease the amount of solvent? Explain the effect this change will have on M, EN, , and the number of stages required
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


