Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For question 5 the two previous experiments were Experiment 16: Qualitative Analysis of Amines and Amine Unknown, and Experiment 17: Preparation of Methyl Orange. For
For question 5 the two previous experiments were Experiment 16: Qualitative Analysis of Amines and Amine Unknown, and Experiment 17: 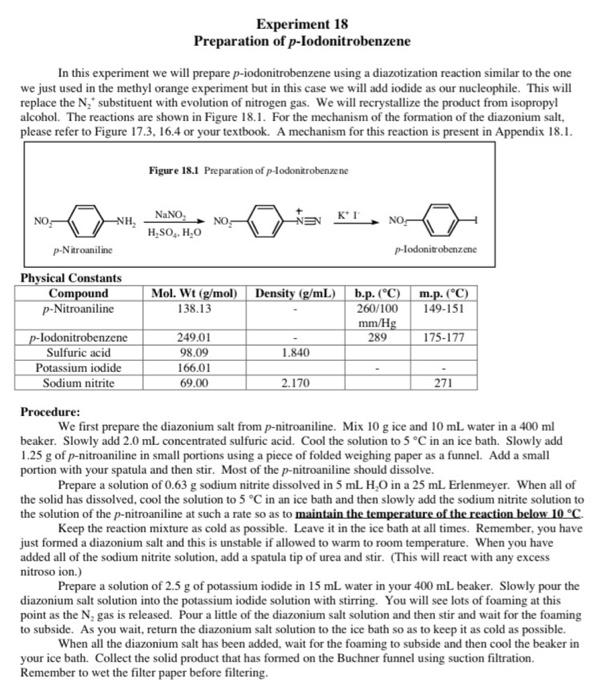

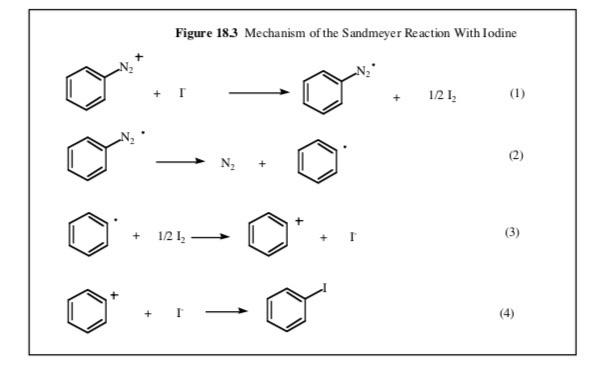


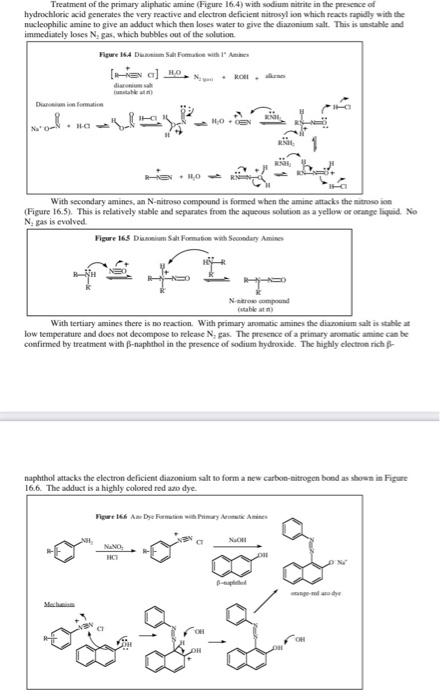
5. In the previous two experiments we had made diazonium salts but did not add any urea at the end. Why is urea needed in this experiment but not in the previous two? 6. The following weights and volumes were recorded for this experiment: 15.0 g ice, 15 mL water, 1.5 mL concentrated H.SO,, 0.90g p-nitroaniline, 0.50g NaNO and 2.0g KI. The final yield of recrystallized product was 1.30g. Calculate the percent yield for this experiment. Experiment 18 Preparation of p-lodonitrobenzene In this experiment we will prepare p-iodonitrobenzene using a diazotization reaction similar to the one we just used in the methyl orange experiment but in this case we will add iodide as our nucleophile. This will replace the N, substituent with evolution of nitrogen gas. We will recrystallize the product from isopropy! alcohol. The reactions are shown in Figure 18.1. For the mechanism of the formation of the diazonium salt, please refer to Figure 17.3. 16.4 or your textbook. A mechanism for this reaction is present in Appendix 18.1. Figure 18.1 Preparation of plodonitrobenzene KI NO NH, NaNO H.SO, H,0 NOS NO- p-Nitroaniline p-lodonitrobenzene Physical Constants Compound p-Nitroaniline p-lodonitrobenzene Sulfuric acid Potassium iodide Sodium nitrite Mol. Wt (g/mol) Density (g/mL) b.p. (C) m.p. (C) 138.13 260/100 149-151 mm/Hg 249.01 289 175-177 98.09 1.840 166.01 69.00 2.170 271 Procedure: We first prepare the diazonium salt from p-nitroaniline. Mix 10 g ice and 10 ml water in a 400 ml beaker. Slowly add 2.0 mL concentrated sulfuric acid. Cool the solution to 5 C in an ice bath. Slowly add 1.25 g of p-nitroaniline in small portions using a piece of folded weighing paper as a funnel. Add a small portion with your spatula and then stir. Most of the p-nitroaniline should dissolve. Prepare a solution of 0.63 g sodium nitrite dissolved in 5 mL H0 in a 25 mL Erlenmeyer. When all of the solid has dissolved. cool the solution to 5 C in an ice bath and then slowly add the sodium nitrite solution to the solution of the p-nitroaniline at such a rate so as to maintain the temperature of the reaction below 10 C Keep the reaction mixture as cold as possible. Leave it in the ice bath at all times. Remember, you have just formed a diazonium salt and this is unstable if allowed to warm to room temperature. When you have added all of the sodium nitrite solution, add a spatula tip of urea and stir. (This will react with any excess nitroso ion.) Prepare a solution of 2.5 g of potassium iodide in 15 mL water in your 400 ml beaker. Slowly pour the diazonium salt solution into the potassium iodide solution with stirring. You will see lots of foaming at this point as the N, gas is released. Pour a little of the diazonium salt solution and then stir and wait for the foaming to subside. As you wait, return the diazonium salt solution to the ice bath so as to keep it as cold as possible. When all the diazonium salt has been added, wait for the foaming to subside and then cool the beaker in your ice bath. Collect the solid product that has formed on the Buchner funnel using suction filtration. Remember to wet the filter paper before filtering. Recrystallize your product from isopropyl alcohol using approximately 20 ml/g of product. You will need around 50 mL in total but do not add all of this at once. Add about 2/3 of the projected amount, heat to boiling using a water bath and then continue adding isopropyl alcohol in small amounts (5-7 mL) until all of the material dissolves. Collect the recrystallized product by suction filtration on the Buchner funnel (wet the filter paper this time with isopropyl alcohol, not water), weigh your sample and submit it in a vial. Calculate the percent yield. take the melting point range and turn in your Organic Yield Report Sheet. Appendix 18.1 Though most text books state that the mechanism for these reactions is not fully understood, a plausible mechanism is provided by Professor Zavitsas in Figure 18.2. This is a chain reaction, not in radicals, but in copper. Figure 18.2 Mechanism of the Sandmeyer Reaction + Cux Cu" x (1) () ) 2) Cu (3) () ( (4) Actually, steps 3 and 4 are one step, a ligand transfer (3.4) CuX With iodine you don't need the copper to be both an oxidizing and a reducing agent. The redox potentials of the I/1, couple are just right to do both the oxidation and the reduction (see Figure 18.3). Figure 183 Mechanism of the Sandmeyer Reaction with lodine + 1 121, (1) () (2) + 1212 (3) + (4) Experiment 17 Preparation of Methyl Orange the people thamaattil magmatise mills said indicato pure 17. The animiseende form is not You will then mogelig i coupling reaction just like the one you the per tomatic in the evaluaciones Fie de meci in figure 173 All of this chemiery is your best in the clapeta Yondant NA The first unrisips are he was als tamil silt in itle w walce. There were the by the that este in Figure 164 the experiem where the same from Note this reacts with the amintom the ones water and the Armenia ante de este de Il can be attacked by which is al blen wat het my Sean bydrae entradas em anime of the N.N dimineata come people de la the activating effect of the dimethylamine Anack is in power lindrance at the lepim by the bulky dmclient SOOS > 1 tota 101 kamera 17 to - Detail Experimeat 16 Qualitative Analysis of Amines and Amine ko In this experiment with Hamberg Teleseriedai between primary day and try to Weather we will consify an area of the recepten Werthely in urdinate Primary.comity and yellow bone per a les the reci Axwe expectantines are and cleopatie. They of the price racking electrophile tracticipheringed by the electrophilic foldlede die Arbeitene weget med en met een sutice. The beneficiaries The them hydropens the nitrogen in de and is led to add the imagine delal in pecies that soluble in watt. Note de este siti Sad HORRO o Withcredary amines are 1625. then the peale amine. This species in mobile in and does the abdomes comienie oso give a soluble pecies. The present the oluble in an acidic solution since the lower pacte aminek egy witdusing atleast Totiary escala de dependiente da se det able come with it and management will forman egen er en sap of the water. They were headed -: 010-4K HORO Treatment of the primary aliphatic amine (Figure 16.4) with sodium nitrite in the presence of hydrochloric acid generates the very reactive and clectron deficient nitrusyl ion which reacts rapidly with the nucleophilic amine to give an adduct which then loses water to give the diazonium salt. This is stable and immediately loses Ngas, which bubbles out of the solution Figure 164 Domates ( a) HD ROM dim Dainformation HOEN . With secondary amines, an N-nitroso compound is formed when the amine attacks the nitroson Figure 16.5). This is relatively stable and separates from the aqueous solution as a yellow or orange liquid. No Nas is evolved Figure 165 Salt Formation with Sendary Amines Ne compound (stable) With tertiary amines there is no reaction. With primary aromatic amines the diaronium salt is stable low temperature and does not decompose to relcase N, gas. The presence of a primary aromatic amine can be confirmed by treatment with B-naphthol in the presence of sodium hydroxide. The highly electronich naphthot attacks the electron deficient diazonium salt to form a new carbco-nitrogen bond as shown in Figure 16.6. The adduct is a highly colored redazo dye Figure 166 An Dewi Primary Am X NAND e tyre dhe Mac Preparation of Methyl Orange.
For question 6 its on Experiment 18: Preparation of p-Iodonitrobenzene
Thank You. :) 







Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


