Answered step by step
Verified Expert Solution
Question
1 Approved Answer
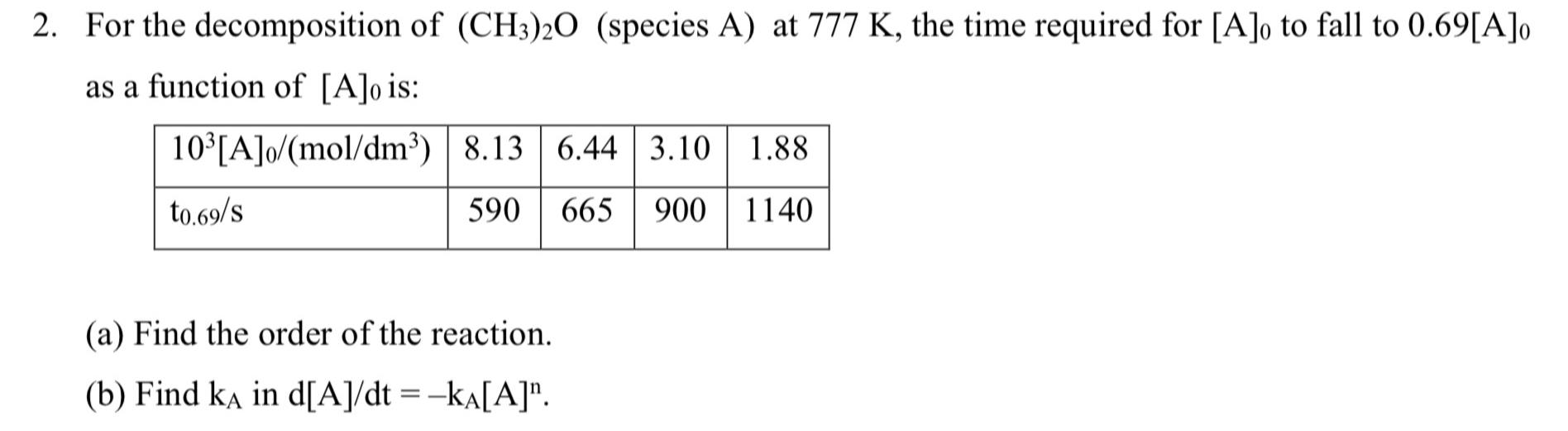
For the decomposition of (CH_(3))_(2)O (species { ( :A) } at 777K , the time required for [A]_(0) to fall to 0.69[A]_(0) as a function
For the decomposition of
(CH_(3))_(2)O(species
{(
:A)} at
777K, the time required for
[A]_(0)to fall to
0.69[A]_(0)as a function of
[A]_(0)is:\ \\\\table[[
10^(3)([A]_(0))/(mo(l)/(d)m^(3)),8.13,6.44,3.10,1.88],[
(t_(0.69))/(s),590,665,900,1140]]\ (a) Find the order of the reaction.\ (b) Find
k_(A)in
d(A)/(d)t=-k_(A)[A]^(n).
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


