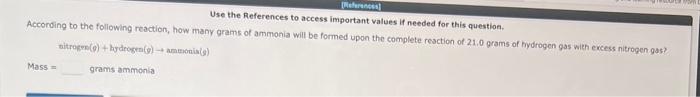
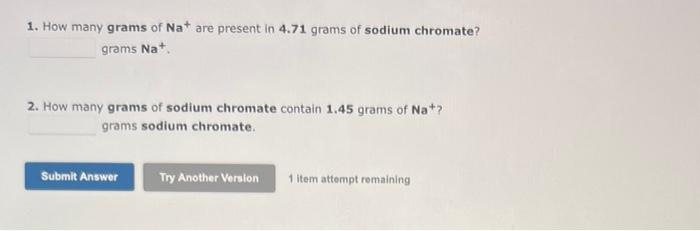
For the following reaction, 0.335 moles of potassium hydrogen sulfate are mixed with 0.543 moles of potassium hydroxide. potassium hydrogen sulfate (aq)+ potassium hydroxide (aq) potassium sulfate (aq)+water() What is the formula for the limiting reagent? Umiting reagent: What is the maximum amount of potassium sulfate that can be produced? Amount = moles 1 item attempt romaining According to the following reaction, how many moles of carbon dioxide will be formed upon the complete reaction of 23.4 grama of carbon (graphite) with excess oxygen gas? carbon (graphite) (s)+ oxygen (g) carbon dioxide (g) moles carbon dioxide The Illustration to the left represents a mixture of phosphorus (orange) and fluorine (green) molecules. If the molecules in the above illustration react to form PF3 accordi P4+6F24PF3 The illustration on the left represents a mixture of Xe (brown) atoms and F2 (green) molecules. If these were to react to form XeF4 molecules, what is the maximum number of XeF4 molecules that couid form? Hint: Atoms must be conserved in an ordinary chemical reaction. Use the References to access important values if needed for this question. According to the following reaction, how many grams of ammonia will be formed upon the complete resction of 21.0 grams of hydrogen gas with excess nitrogen gas? titrogen(9)+hydrogkn(g)anmonis(g)Mass=gramsammonia According to the following reaction, how many moles of ammonium nitrite are necessary to form 0.766 moles water? ammonium nitrite (aq) nitrogen (g)+ water (I) moles ammonium nitrite 1 item attempt remaining When the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coeffients are: phosphorus (P4)(s)+ chlorine (g) phosphorus trichloride (I) Consider the following unbalanced particulate representation of a chemical equation: Q+QQ&I=purpleF=green Write a balanced chemical equation for this reaction, using the smallest integer coefficients possible. Do not include states. 1. How many grams of Na+are present in 4.71 grams of sodium chromate? grams Na+. 2. How many grams of sodium chromate contain 1.45 grams of Na+? grams sodium chromate. The percent by mass of chlorine in B5H8Cl is %. (Enter your answer to four significant figures.) a. How many ATOMS of silicon are present in 3.88grams of silicon dioxide? atoms of silicon. b. How many GRAMS of oxygen are present in 3.381022 molecules of silicon dioxide? grams of oxygen