Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For the oxidation of ethylene to Ethyleneoxide, the feed stream contains ethylene: oxygen in a stoichiometric molar ratio of 2:1. The properties of the mixture
For the oxidation of ethylene to Ethyleneoxide, the feed stream contains ethylene: oxygen in a stoichiometric molar ratio of 2:1. The properties of the mixture and pure components are given below depending on the Head at a constant temperature of 0 oC.
a) Calculate the fugacity of oxygen and ethylene in the mixture at T= 00C and P= 25 atm, based on the Lewis-Randall rule.
b) Calculate the fugacity of oxygen and ethylene in the mixture at T= 00C and P= 25 atm using the data given below.
| P (atm) | 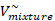 M3/kmol M3/kmol | 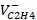 M3/kmol M3/kmol | 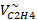 M3/kmol M3/kmol | 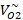 M3/kmol M3/kmol |
| 1 | 22,256 | 22,211 | 22,189 | 22,350 |
| 5 | 4,366 | 4,323 | 4,294 | 4,456 |
| 10 | 2,129 | 2,087 | 2,053 | 2,219 |
| 15 | 1,384 | 1,342 | 1,305 | 1,473 |
| 20 | 1,011 | 0,970 | 0,928 | 1,100 |
| 25 | 0,787 | 0,747 | 0,700 | 0,877 |
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


