Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For the second set of problems, match the blue square on the left to it's corresponding blue square on the right. Multiple squares can have
For the second set of problems, match the blue square on the left to it's corresponding blue square on the right. Multiple squares can have the same answer. I can't seem to figure these out so help would be appreciated. Thank you in advance!
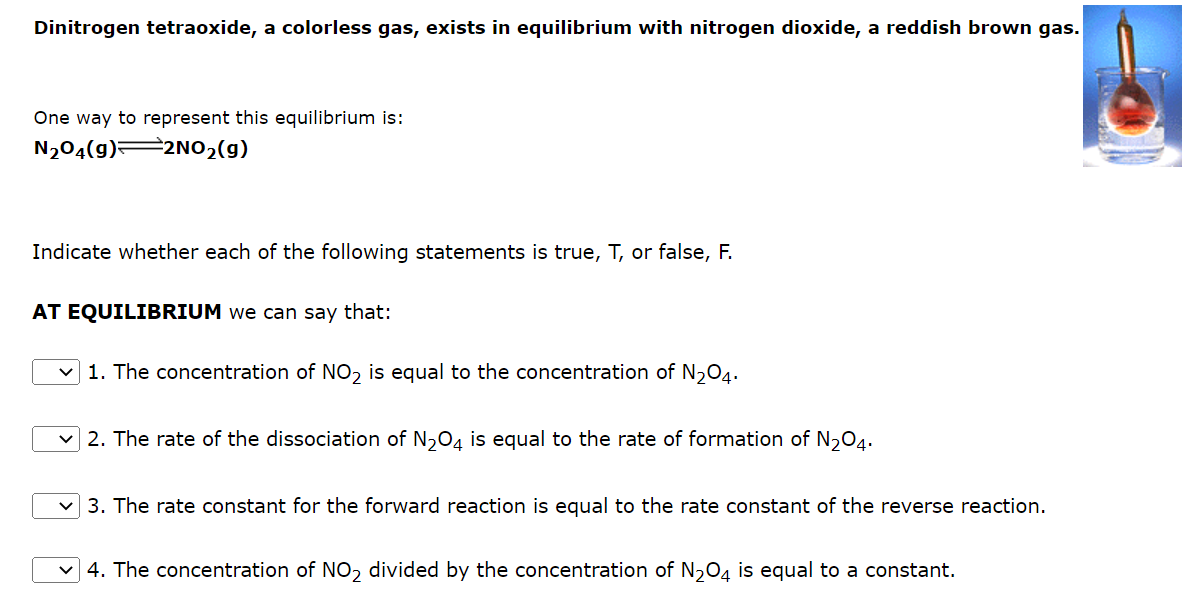
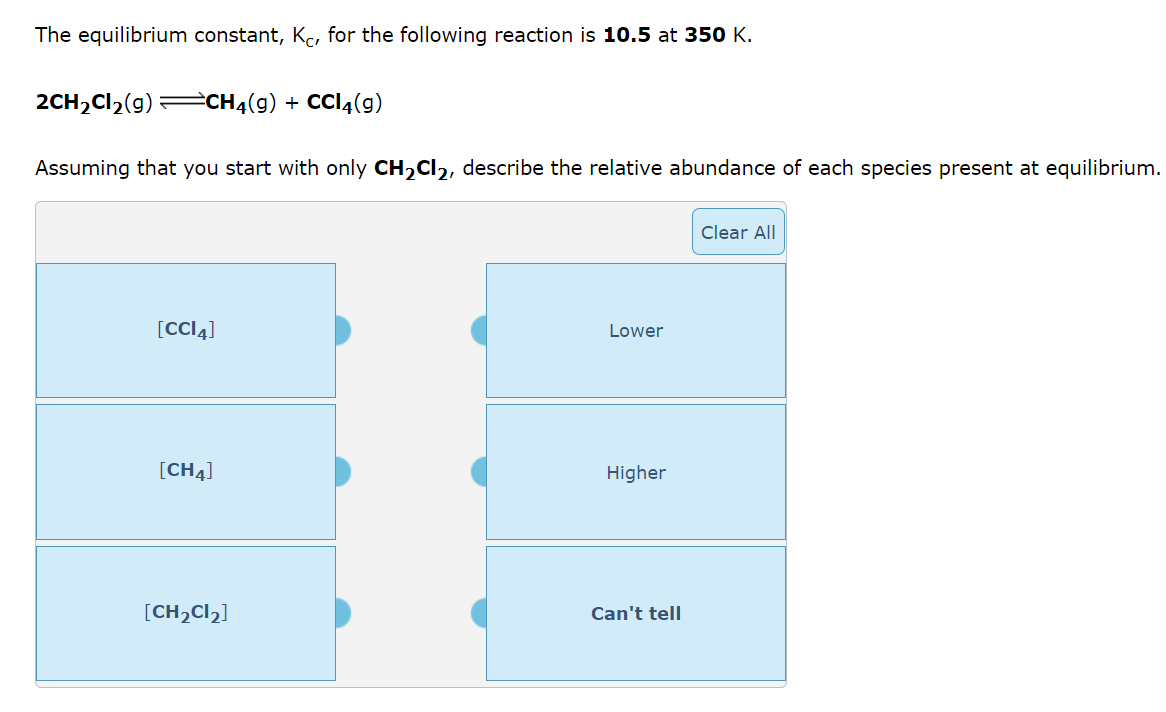
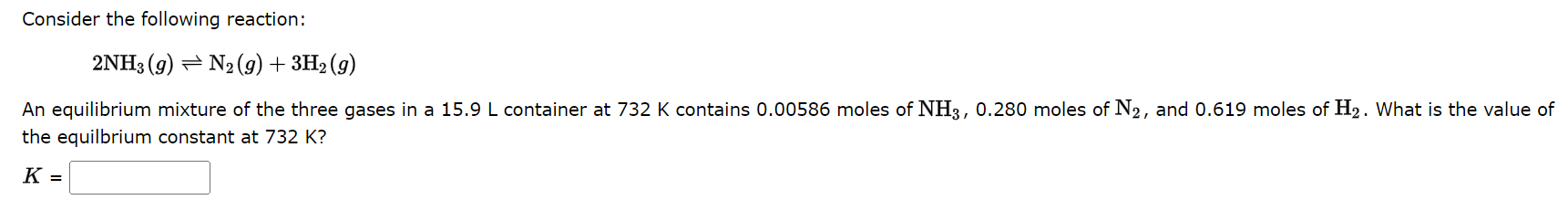
Dinitrogen tetraoxide, a colorless gas, exists in equilibrium with nitrogen dioxide, a reddish brown gas One way to represent this equilibrium is: N2O4(g)2NO2(g) Indicate whether each of the following statements is true, T, or false, F. AT EQUILIBRIUM we can say that: 1. The concentration of NO2 is equal to the concentration of N2O4. 2. The rate of the dissociation of N2O4 is equal to the rate of formation of N2O4. 3. The rate constant for the forward reaction is equal to the rate constant of the reverse reaction. 4. The concentration of NO2 divided by the concentration of N2O4 is equal to a constant. The equilibrium constant, Kc, for the following reaction is 10.5 at 350K. 2CH2Cl2(g)CH4(g)+CCl4(g) Consider the following reaction: 2NH3(g)N2(g)+3H2(g) An equilibrium mixture of the three gases in a 15.9L container at 732K contains 0.00586 moles of NH3,0.280 moles of N2, and 0.619 moles of H2. What is the value of the equilbrium constant at 732K ? K=
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


