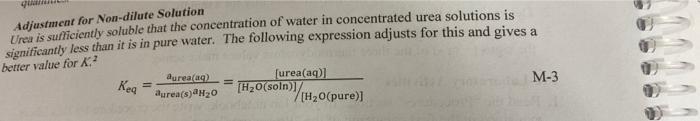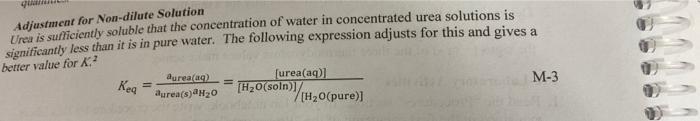for this lab i need to find the delta H, delta G, snd delta S. I dont even know where to begin.
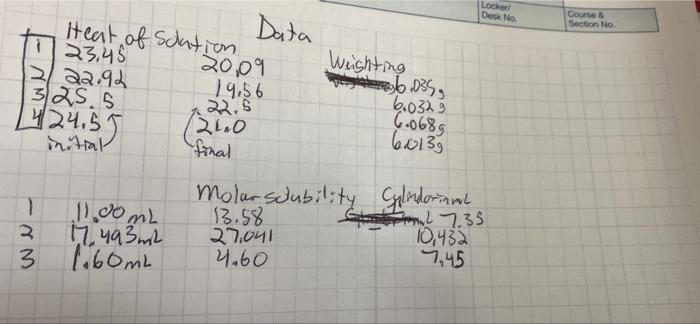
Calculations Project M. Thermodynamics quantities and the maximum temperature change. Set up a clear sample calculation for the enthalpy of solution from your measured Set up a clear sample calculation of the equilibrium constant, using equation M-3. Set up a clear sample calculation to determine each of the thermodynamic quantities for dissolution of urea: AHm AG, and AS, where the m indicates molar quantities, e.g. in m. kJ/mole. Adjustment for Non-dilute Solution Urea is sufficiently soluble that the concentration of water in concentrated urea solutions is significantly less than it is in pure water. The following expression adjusts for this and gives a better value for X.? aureaa2 [urea(aq)] aurea(s)H20 [H2O(soln/ M-3 [H2O(pure)] Keg Locker Deck No Data Course Section No. . s Heat of Sclation 23,45 20,09 Weighting 2/ 22.93 19,56 | .035, 325.5 6.0329 24 24.55 ( 6.068g 20.0 ( 60139 initial final Molar solubility Glendarium 1 11.00ml 3.5 SL 7.35 3. 10,432 17. 493 mil 27.041 4.60 7,45 3 1.60mL we- Project 1194-M Determination of Thermodynamic Quantities Objectives 1. Determine an approximate equilibrium solubility for a rapidly dissolving substance. 2. Determine the thermodynamic quantities for that process: AH, AG', and AS 3. Identify the driving force for the formation of a solution. Think about results and determine if the signs (+ or -) of the three thermodynamic values make sense. Pre-Laboratory Activities 1. Read the Procedures" section. In your lab notebook, write the procedure for this lab as explained in the first paragraph of the Procedures" section. 2. Review thermochemistry (enthalpy) from Chem 1180 and your lecture textbook, and your work in Chem 1184 Project M. Plan how you will use laboratory measurements to determine the quantities AH", AG", and As. Review your lecture textbook for the thermodynamic equations to be used. Look for relationships to other physical properties that are measured. 3. Find the molar mass for urea and draw the Lewis structure of urea. 4. () Is urea a polar or non-polar molecule? (b) Can this molecule hydrogen bond with water? If so, draw a hydrogen bond between the Lewis structures of urea and water 5. Calculate the molarity of pure water at 25C. 6. A 10.0 g piece of aluminum at 100.0C is placed into 40.0 g of water (specific heat of water is 4.18 J/gC) originally at 25.0C. The final temperature of the water is 28.8C. Calculate the heat change (q) of the aluminum (be careful of the sign)? Safety - Find the safety/disposal concern for urea and its aqueous solutions. Background The solubility of a substance which does not dissociate in solution is effectively an equilibrium constant. For example the equilibrium equation for dissolution of urea is urea(s) + H2O(l) urea(aq). M-1 The full equilibrium constant expression (the product of activities of reaction products over the product of activities of reactants) reduces, in concentration terms, to the molar concentration of solute in the saturated solution aurea) = [urea(aq)] Bureau M-2 The activities (effective concentrations) of urea in its pure, solid state and of water in its pure liquid state are constant, regardless of the quantities present. These are the standard states! of these substances. Their activities have the unitless value 1; they disappear from the usual equilibrium constant expression Keq = This work takes place near 25C, the standard temperature, we shall ignore any difference from this. 164 Chemistry 1194 There is no tendency to net reaction at equilibrium. The free energy of products equals that of the reactants; AGES). This gives an entry to the determination of thermodynamic quantities. Adjustment for Non-dilute Solution significantly less than it is in pure water. The following expression adjusts for this and gives a Urea is sufficiently soluble that the concentration of water in concentrated urea solutions is better value for K? Dura= Kegt furea(aq)] [H2O(soin) (120(pure) M-3 aurea 20 Procedures Work out your procedure in groups of two or three, which should be specific as to masses and volumes to be measured as well as data to be taken. For Experiment 1, you will use a coffee-cup calorimeter (approximately 6 oz capacity), available in the lab with a thermometer or digital thermometer. Determine the volume of liquid you want to have in the cup to have rapid dissolution without spilling. In addition, for each of the two experiments below you should have a data table with the necessary columns in the "Data" section of your lab notebook. These data tables should also have multiple rows for each of the trials you will perform 6,032-6035 Experiment 1: Heat of Solution. Because I M is a standard state concentration, and preparation of a solution at this concentration should give enough temperature change for decent precision, use this concentration. Weigh the appropriate mass of urea and record this mass. Measure the mass or volume of pure water. Record the initial temperature (T) of the water in the calorimeter before adding the urea. Use a steady rate of stirring to mix the solid urea into the water in the calorimeter. Monitor the temperature over time to determine the maximum temperature change and record the final temperature (Tr) of the calorimeter and calculate the temperature change (AT) of the solution (be careful of sign). Specific heat capacity varies with temperature and solution concentration, but through the range of temperatures and concentrations you'll get, the specific heat of the urca solution is expected to be close enough to that of pure water, 4.184 Jg' K-that you should use that value (and ignore the heat capacity of the calorimeter, thermometer, and stirrer), Experiment 2: Molar Solubility. For measurement of molar solubility, you will need to know the volume of saturated solution. Weigh about 4 g of urea using the top loading balance and record this exact mass. Fill a buret with distilled water. Record the initial buret volume. Add about 1 mL of water from the buret to a 10 ml graduated cylinder. Then transfer the 4 grams of urea to the cylinder and mix with a stirring rod. Next add as much water from the buret as required to barely dissolve all the urea at room temperature. This will be done by adding small portions of water and mixing after each portion is added. When the urea has entirely dissolved, stop adding water, Record the final buret volume and the final saturated solution volume of the graduated cylinder. Use the initial and final buret volumes to determine the volume of water that was required to make that saturated solution. Share and/or repeat measurements in your group to have sufficient data to determine a standard deviation of the results. 27.55. Toject M. Thermodynamics Calculations kJ/mole. Set up a clear sample calculation for the enthalpy of solution from your measured quantities and the maximum temperature change. Set up a clear sample calculation of the equilibrium constant, using equation M-3. Set up a clear sample calculation to determine each of the thermodynamic quantities for dissolution of urea: AH.. AGm, and AS, where the m indicates molar quantities, eg. in students in the class and use these replicates to determine a best value and standard deviation for Collect and tabulate at least three values determined by members of your group or other for those who are interested? Discussion Answer objective #3 using complete sentences. Report Submit your legible notebook carbon copies. These will be evaluated for:1) completeness of data, i) clarity of data labels, oily organization of data, results, and labels, in tables or pattern, iv) correct precision and use of significant figures, v) correct unit labels in sample calculations, vi) correct calculations. You are reporting several values and their standard deviations. To the extent members of a group use the same data, their results will be the same. To the extent that each member checks the work rather than copies that of another, all should be correct Clean Up Discard urea solutions to the container provided. Clean the glassware with soap and rinse with tap and distilled water to minimize beading and spotting later. 3 Calculated by propagation of uncertainty rules, the standard deviation for AS. (Stdev + (Stdevam T