Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Four cubes - one each of aluminum (Al), copper (Cu). iron (Fe), and lead (Pb) - all having the same mass, are heated. The
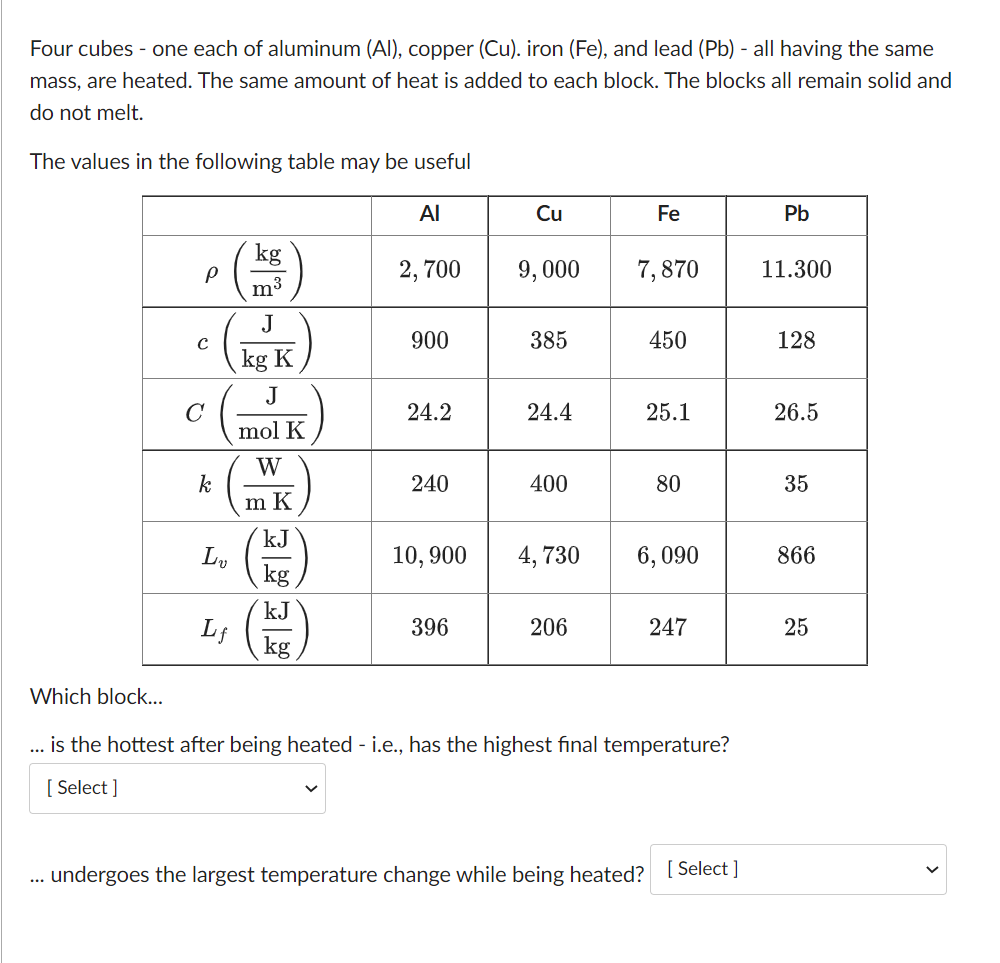
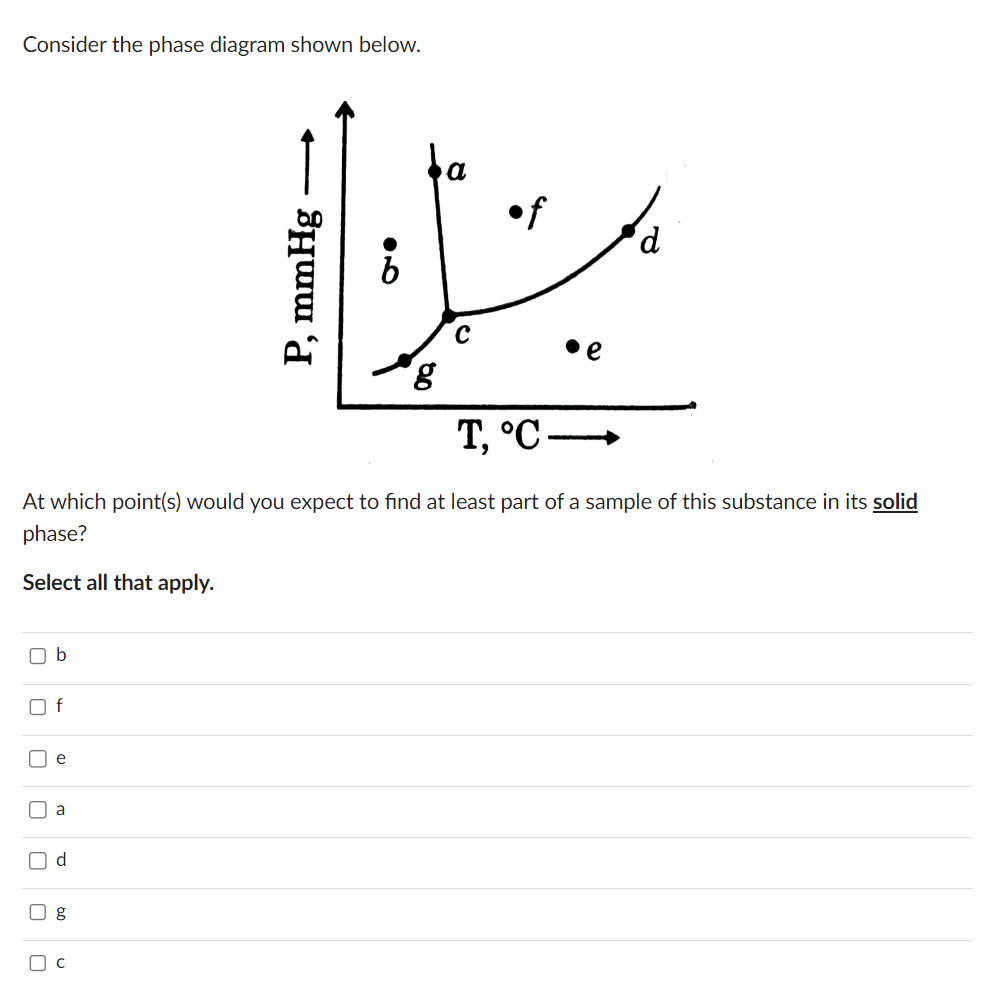
Four cubes - one each of aluminum (Al), copper (Cu). iron (Fe), and lead (Pb) - all having the same mass, are heated. The same amount of heat is added to each block. The blocks all remain solid and do not melt. The values in the following table may be useful Cu Fe Pb kg P 2,700 9,000 7,870 11.300 m J 900 385 450 128 kg K J 0 24.2 24.4 25.1 26.5 mol K W k 240 400 80 35 35 m K kJ Lv 10,900 4,730 6,090 866 kg kJ Lf 396 206 247 25 kg Which block... is the hottest after being heated - i.e., has the highest final temperature? [Select] undergoes the largest temperature change while being heated? [Select] Consider the phase diagram shown below. - P, mmHg 200 a of 20 T, C. At which point(s) would you expect to find at least part of a sample of this substance in its solid phase? Select all that apply. b f U U e a d g ( C
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To solve this problem we need to focus on the specific heat capacity c of each metal and understand ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


