Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Four resonance structures of the following cation are possible.Two resonance forms are given below, but they are incomplete.Complete the given structures by adding nonbonding electrons
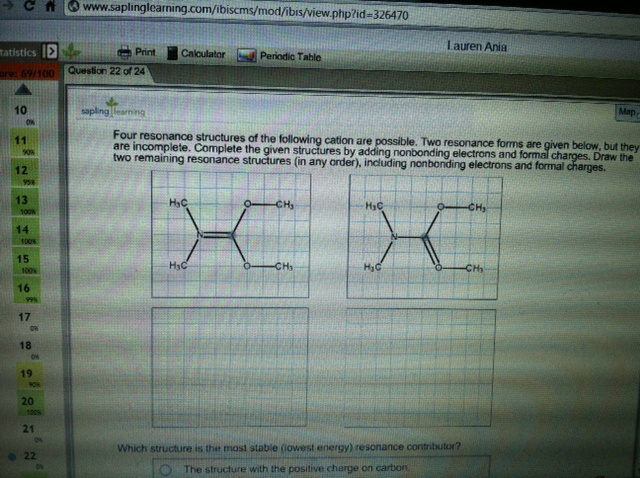
Four resonance structures of the following cation are possible.Two resonance forms are given below, but they are incomplete.Complete the given structures by adding nonbonding electrons andformal charges. Draw the two remaining resonance structures (in anyorder), including nonbonding electrons and formal charges. alsowhich structure is the most stable?
C www.saplinglearning.com/ibiscms/mod/ibis/view.php?id=326470 tatistics > Print ore: 69/100 Question 22 of 24 10 0x 11 90% 12 958 13 100k 14 1006 15 1000 16 99% 17 0% 18 OK 19 90% 20 100% 21 22 ON Calculator HC Periodic Table sapling learning Four resonance structures of the following cation are possible. Two resonance forms are given below, but they are incomplete. Complete the given structures by adding nonbonding electrons and formal charges. Draw the two remaining resonance structures (in any order), including nonbonding electrons and formal charges. HC -CH CH HC Lauren Ania HC Which structure is the most stable (lowest energy) resonance contributor? The structure with the positive charge on carbon -CH Map CH
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The four resonance structures of the compound are shown below CH3 CH3 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


