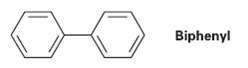
Using resonance structures of the intermediates, explain why bromination of biphenyl occurs at ortho and Para positions
Question:
Using resonance structures of the intermediates, explain why bromination of biphenyl occurs at ortho and Para positions rather than at Meta.
Transcribed Image Text:
Biphenyl
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
Resonance structures explain that bromination occurs in the ortho and Para pos...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Draw both resonance structures of the enolate formed when each of the following ketones is treated with a strong base: (a) (b) (c) (d) (e)
-
Draw resonance structures of the intermediate carbocations in the bromination of naphthalene, and account for the fact that naphthalene undergoes electrophilic substitution at C1 rather than C2. Br...
-
(a) Draw two resonance structures of the cation shown below, shifting only one electron pair in each step.- Be sure to include the formal charge on structures B and C. Only move one double bond. Each...
-
Are there accomplishments for which you individually receive credit which others helped you accomplish?
-
Assume that in addition to the information in Problem 49, Rick and Sara had no taxable income for 2011, 2012, 2013, and 2014 and $3,700 of taxable income for 2015 computed as follows: a. Determine...
-
Beasley Realty Group had two completed unsold buildings on hand on January 1, 2019. Unit 06-92: Cost, $395,000; retail price, $482,000 Unit 06-94: Cost, $436,500; retail price, $504,000 During the...
-
What are the three possible classifications of long-term equity investments? Describe the criteria for each class and the method used to account for each. AppendixLO1
-
You are told the column totals in a trial balance are not equal. After careful analysis, you discover only one error. Specifically, a correctly journalized credit purchase of an automobile for...
-
Introduction to marketing question Research the major factors that contributed to making WALMART the retailing global leader. should be composed within 250 words with APA referencing.
-
A drive has the following parameters: J=10 (kg-m 2 ), T=100-0.1N (N-m), Passive load torque T l =0.05N (N-m), where N is the speed in rpm. Initially the drive is operating in steady-state. Now it is...
-
The nitroso group, N = O, is one of the few non-halogens that is an ortho- and Para-directing deactivator. Explain by drawing resonance structures of the carbocation intermediates in ortho, Meta and...
-
At what position and on what ring do you expect nitration of 4-bromo- biphenyl to occur? Explain, using resonance structures of the potentialintermediates. 4-Bromobiphenyl Br
-
The weekly demand and supply schedules for t-shirts (in millions) in a free market are as follows: Price () 8 7 6 5 4 3 2 1 Quantity demanded 6 8 10 12 14 16 18 20 Quantity supplied 18 16 14 12 10 8...
-
Convex Productions produces full-length motion pictures for distribution worldwide. Convex has just purchased the rights to a movie script entitled Native Sun, which it intends to develop as its next...
-
You are visiting the Engineering Office of Denton Hospital, as part of a consulting project. You notice some charts on one wall which look familiar to you: One of the employees notices you reading...
-
Richmond Clinic has obtained the following estimates for its costs of debt and equity at different capital structures: What is the firms optimal capital structure? (Hint: Calculate its corporate cost...
-
Suppose a sample yields estimates \(\widehat{\theta}_{1}=5, \widehat{\theta}_{2}=3\), se \(\left[\widehat{\theta}_{1} ight]=2\), and se \(\left[\widehat{\theta}_{2} ight]=1\) and the correlation...
-
Helium expands in a nozzle from \(0.8 \mathrm{MPa}, 500 \mathrm{~K}\), and negligible velocity to \(0.1 \mathrm{MPa}\). Calculate the throat and exit areas for a mass flow rate of \(0.34 \mathrm{~kg}...
-
How could managers approach these circumstances ethically?
-
a) Show that (a, b) := {{a}, {b}} does not satisfy the ordered pair axiom. b) Determine whether each of the following statements is true or false. (Give a reason in each case): (i) {a, b} C (a, b)....
-
In the calculation of thermodynamic properties, it is convenient to have the following partial derivatives: where Z = (P V /RT) is the compressibility factor. Develop expressions for these two...
-
Arrange these alkenes in order of increasing rate of reactio0n with HCI: CH, CH2=CH2 a) CH;CH2CH=CH2 CH;CH,C=CH, -CH=CH; CH=CH2 CH,CH-CH b) CHO
-
Show the structure of the carbocations that are formed in the reaction of HBr with 2-hexena and explain why two products are formed.
-
Show the products of thesereactions: + HCI b) + HF + HI CH3 + HBr CH3 + HCI CH,CH, (p)
-
Corom Stack Standard CALCULATOR PRINTER VERSION BACK NEXT Problem 13-02A a-c (Part Level Submission) Sheffield Corporation had the following stockholders' equity accounts on January 1, 2020: Common...
-
Suppose that you own 2,100 shares of Nocash Corp. and the company is about to pay a 25% stock dividend. The stock currently sells at $115 per share. a. What will be the number of shares that you hold...
-
Diplomatic Security Service provides Airport Transportation and Surveillance Service to Foreign Diplomats in Guyana. The company has two support departments - Information Systems and Equipment...

Study smarter with the SolutionInn App


