Answered step by step
Verified Expert Solution
Question
1 Approved Answer
fourth part (6 pts.) J) Calculate the bulk density of B-Mg Te in grams per cubic centimeter. The atomic masses of Mg and Te are
fourth part 
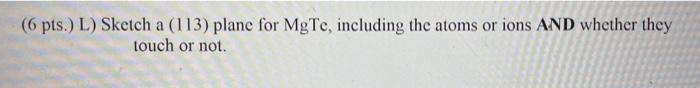
(6 pts.) J) Calculate the bulk density of B-Mg Te in grams per cubic centimeter. The atomic masses of Mg and Te are 24.305 g/mole of atoms and 127.6 g/mole of atoms, respectively. Avogadro's # = 6,022 x 1023 atoms/mole of atoms. (5 pts.) K) Sketch a (113) plane. (6 pts.) L) Sketch a (113) plane for MgTe, including the atoms or ions AND whether they touch or not 
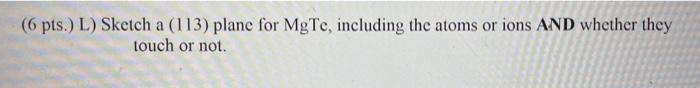
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


