Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Fractional distillation tower is one of the most common unit operations of chemical engineering which is used to separate large quantities of liquids such as
Fractional distillation tower is one of the most common unit operations of chemical engineering which is used to separate large quantities of liquids such as water and ethanol mixture. A simple fractional distillation tower generates two product streams which are called top and bottom from a fresh feed.
Suppose you are working as an engineer in a distillation unit. This unit consists of two distillation towers. A liquid mixture of ethanol, dissolved MgCl salt and water is continuously fed into the first distillation tower. The tower separates the mixture into two product streams which have equal mass flow rates. The top stream contains ethanol by mass and no MgCl The bottom stream is purged.
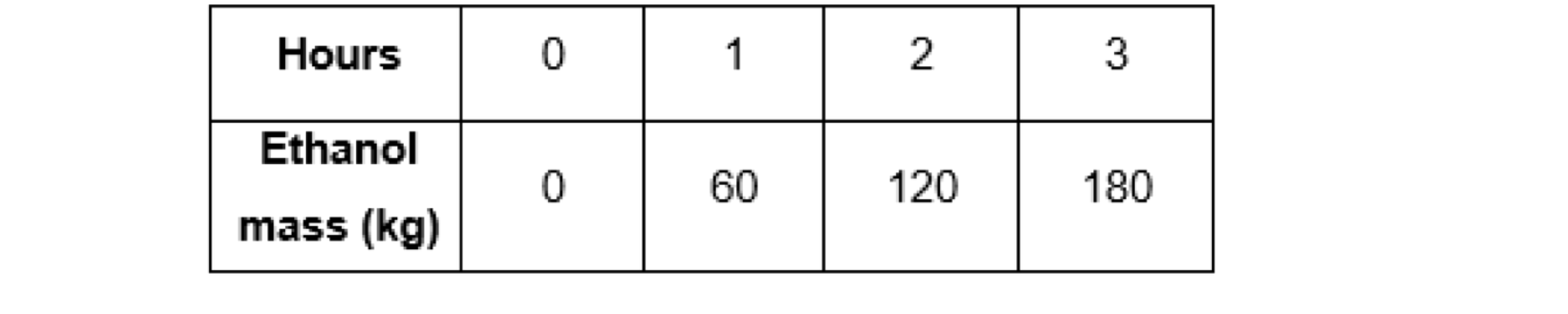
The top stream is then fed into the second distillation tower. This tower generates two streams as well. The top stream consists of pure ethanol, and the bottom stream contains water by mass. The bottom stream is purged again, and the top stream is stored in an ethanol tank. Your job as a chemical engineer is to check if this distillation unit works at steady state, and to perform relevant calculations. To accomplish your job suppose you measure the mass of the pure ethanol accumulated in the ethanol tank. Your collected data is given below.
a Draw the block diagram and label the knowns and unknowns.
b VerifythatthedistillationunitworksatsteadystateHint:Considerthegiven
table
c Calculate the molar flowrate of MgCl that exits the unit in molesh and the
mass flowrate of the fresh feed in kghMgCl gmol
you cant use ethanols s mass flow directly to calculate the feeds flow rate like guys come on you need to do mass or material balance like Ffirst top streamFsecond top streamFfeed Ffirst top streamkghr FF F stuff like this also keeping n mind the mass fractions and percentages
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


