Please calculate how the yellow highlighted is gotten? I am stuck on how these parts got? 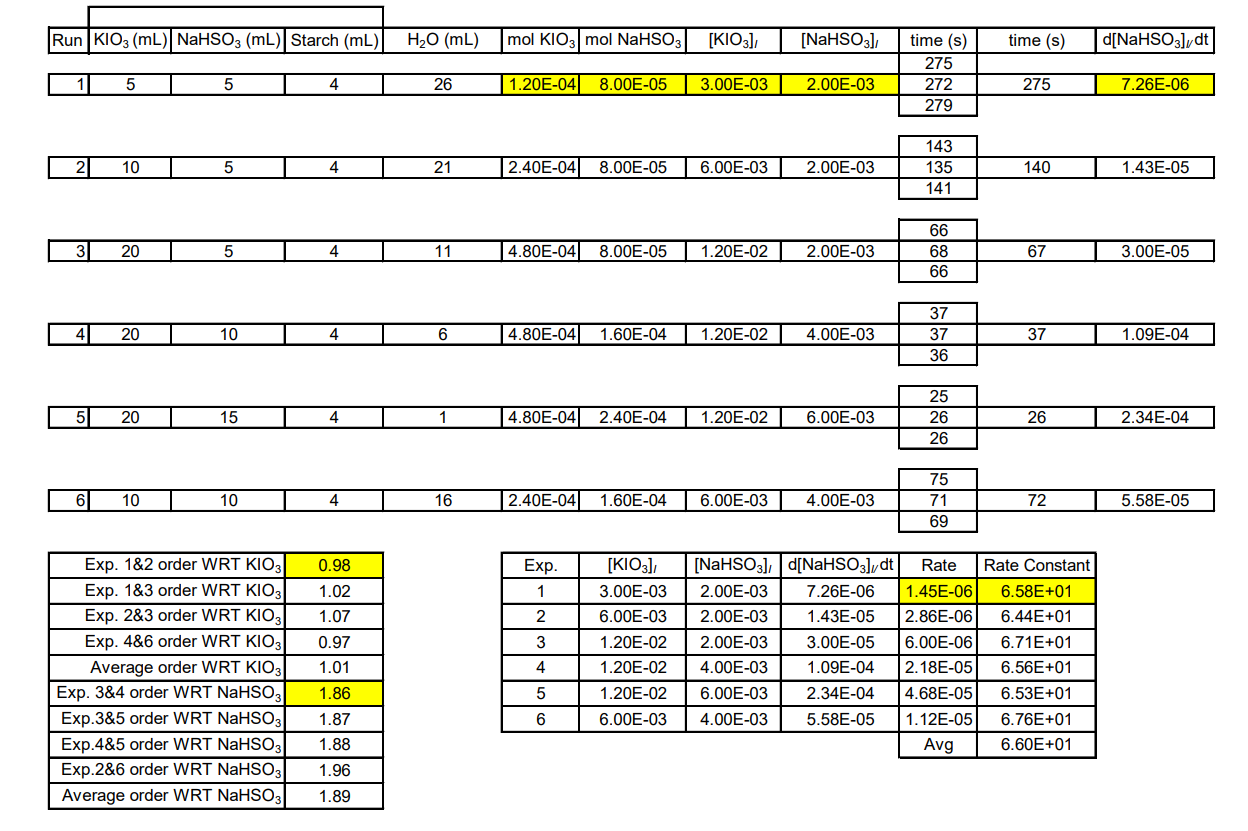
Run KIO3 (mL) NaHSO3 (mL) Starch (mL) H20 (mL) mol KIO3 mol NaHSO3 [KIO3] [NaHSO3] time (s) d[NaHSO3]dt time (s) 275 272 279 1 5 5 4 26 1.20E-04 8.00E-05 3.00E-03 2.00E-03 275 7.26E-06 2 10 5 4 21 2.40E-04 8.00E-05 6.00E-03 2.00E-03 143 135 141 140 1.43E-05 3 20 5 4 11 4.80E-04 8.00E-05 1.20E-02 2.00E-03 66 68 66 67 3.00E-05 4 20 10 4 6 4.80E-04 1.60E-04 1.20E-02 4.00E-03 37 37 36 37 1.09E-04 20 15 4.80E-04 2.40E-04 1.20E-02 6.00E-03 25 26 26 26 2.34E-04 6| 10 10 4 16 2.40E-04 1.60E-04 6.00E-03 4.00E-03 75 71 69 72 5.58E-05 Exp 0.98 1.02 1.07 1 2 3 Exp. 1&2 order WRT KIO3 Exp. 1&3 order WRT KIO3 Exp. 2&3 order WRT KIO3 Exp. 4&6 order WRT KIO3 Average order WRT KIO3 Exp. 3&4 order WRT NaHSO3 Exp.3&5 order WRT NaHSO3 Exp.4&5 order WRT NaHSO3 Exp.2&6 order WRT NaHSO3 Average order WRT NaHSO3 [KIO3] 3.00E-03 6.00E-03 1.20E-02 1.20E-02 1.20E-02 6.00E-03 0.97 1.01 1.86 1.87 1.88 4 [NaHSO3] [NaHSO3], dt Rate Rate Constant 2.00E-03 7.26E-06 1.45E-06 6.58E+01 2.00E-03 1.43E-05 2.86E-06 6.44E+01 2.00E-03 3.00E-05 6.00E-06 6.71E+01 4.00E-03 1.09E-04 2.18E-05 6.56E+01 6.00E-03 2.34E-04 4.68E-05 6.53E+01 4.00E-03 5.58E-05 1.12E-05 6.76E+01 Avg 6.60E+01 5 6 1.96 1.89 Run KIO3 (mL) NaHSO3 (mL) Starch (mL) H20 (mL) mol KIO3 mol NaHSO3 [KIO3] [NaHSO3] time (s) d[NaHSO3]dt time (s) 275 272 279 1 5 5 4 26 1.20E-04 8.00E-05 3.00E-03 2.00E-03 275 7.26E-06 2 10 5 4 21 2.40E-04 8.00E-05 6.00E-03 2.00E-03 143 135 141 140 1.43E-05 3 20 5 4 11 4.80E-04 8.00E-05 1.20E-02 2.00E-03 66 68 66 67 3.00E-05 4 20 10 4 6 4.80E-04 1.60E-04 1.20E-02 4.00E-03 37 37 36 37 1.09E-04 20 15 4.80E-04 2.40E-04 1.20E-02 6.00E-03 25 26 26 26 2.34E-04 6| 10 10 4 16 2.40E-04 1.60E-04 6.00E-03 4.00E-03 75 71 69 72 5.58E-05 Exp 0.98 1.02 1.07 1 2 3 Exp. 1&2 order WRT KIO3 Exp. 1&3 order WRT KIO3 Exp. 2&3 order WRT KIO3 Exp. 4&6 order WRT KIO3 Average order WRT KIO3 Exp. 3&4 order WRT NaHSO3 Exp.3&5 order WRT NaHSO3 Exp.4&5 order WRT NaHSO3 Exp.2&6 order WRT NaHSO3 Average order WRT NaHSO3 [KIO3] 3.00E-03 6.00E-03 1.20E-02 1.20E-02 1.20E-02 6.00E-03 0.97 1.01 1.86 1.87 1.88 4 [NaHSO3] [NaHSO3], dt Rate Rate Constant 2.00E-03 7.26E-06 1.45E-06 6.58E+01 2.00E-03 1.43E-05 2.86E-06 6.44E+01 2.00E-03 3.00E-05 6.00E-06 6.71E+01 4.00E-03 1.09E-04 2.18E-05 6.56E+01 6.00E-03 2.34E-04 4.68E-05 6.53E+01 4.00E-03 5.58E-05 1.12E-05 6.76E+01 Avg 6.60E+01 5 6 1.96 1.89







