Answered step by step
Verified Expert Solution
Question
1 Approved Answer
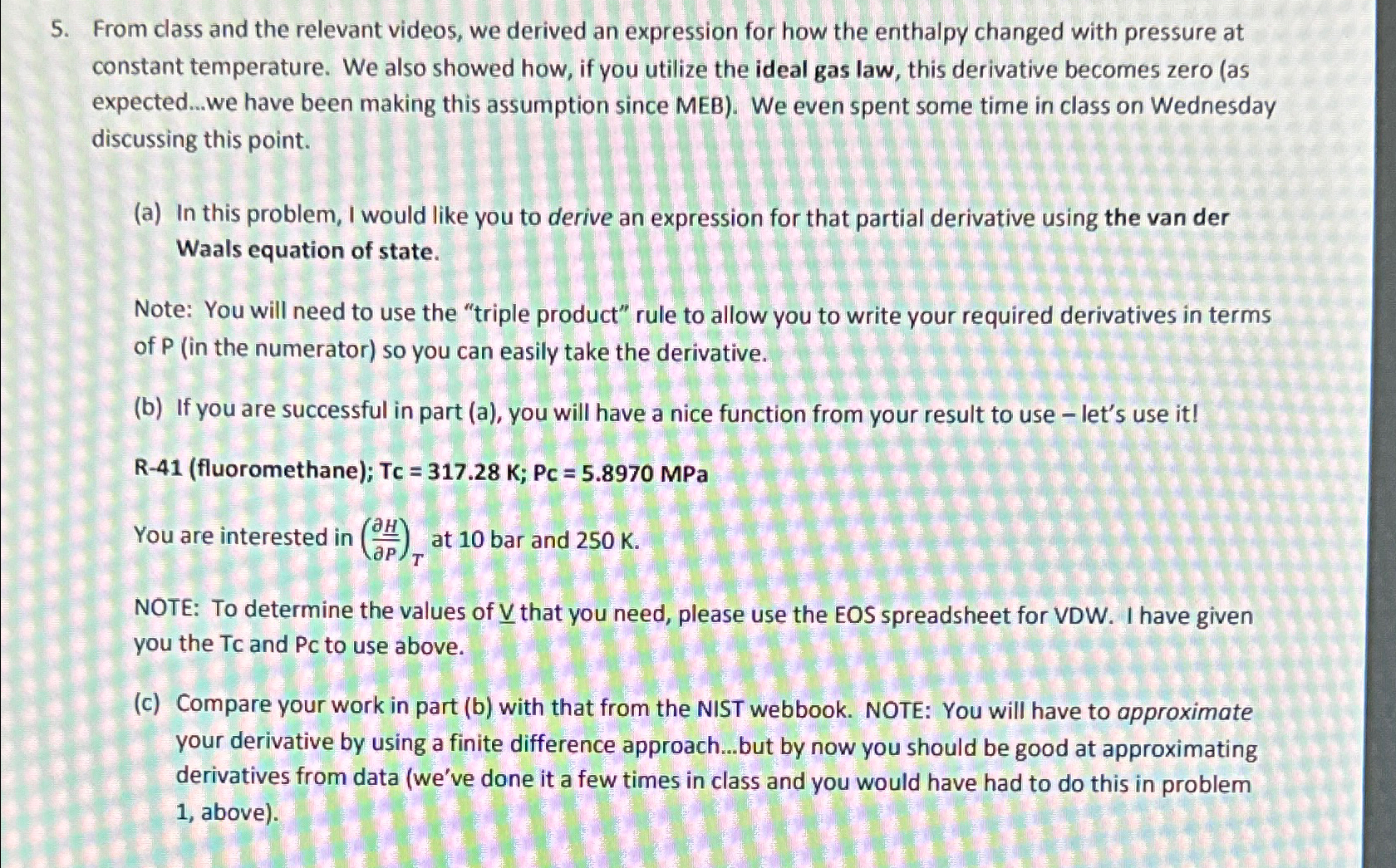
From class and the relevant videos, we derived an expression for how the enthalpy changed with pressure at constant temperature. We also showed how, if
From class and the relevant videos, we derived an expression for how the enthalpy changed with pressure at constant temperature. We also showed how, if you utilize the ideal gas law, this derivative becomes zero as expected...we have been making this assumption since MEB We even spent some time in class on Wednesday discussing this point.
a In this problem, I would like you to derive an expression for that partial derivative using the van der Waals equation of state.
Note: You will need to use the "triple product" rule to allow you to write your required derivatives in terms of in the numerator so you can easily take the derivative.
b If you are successful in part a you will have a nice function from your result to use let's use it Rfluoromethane; Tc ;MPa
You are interested in at bar and
NOTE: To determine the values of that you need, please use the EOS spreadsheet for VDW I have given you the Tc and Pc to use above.
c Compare your work in part b with that from the NIST webbook. NOTE: You will have to approximate your derivative by using a finite difference approach...but by now you should be good at approximating derivatives from data weve done it a few times in class and you would have had to do this in problem above
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


