Answered step by step
Verified Expert Solution
Question
1 Approved Answer
From the following data plot, calculate the activation energy, Ea, for the reaction. How am I supposed to do that? I don't even know what
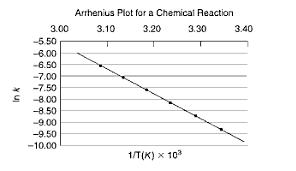
From the following data plot, calculate the activation energy, Ea, for the reaction.
How am I supposed to do that? I don't even know what those points are because they aren't directly at 3.10, etc, it's all aguess...
3.00 -5.50 -6.00 -6.50 -7.00 -7.50 S -8.00 -8.50 -9.00 -9.50 -10.00 Arrhenius Plot for a Chemical Reaction 3.10 3.20 3.30 1/T(K) x 10 3.40
Step by Step Solution
★★★★★
3.51 Rating (168 Votes )
There are 3 Steps involved in it
Step: 1
Answer us K A EART e log K logA EA R dd ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


